1. Background
Coronary artery disease is associated with a high incidence of cardiac events, and drug therapy, dietary management and non-invasive therapies are the mainstay of treatment. However if these methods were not responsive, to resolve problems coronary artery bypass graft surgery is done (CABG) in order to increase survival and improve the quality of patients’ lives (1, 2). There are some early post-operative complications including peri-operative myocardial infraction in CABG in spite of its favorable long term outcome. Cardiac arrhythmias is one of the most common complications after open heart surgery, which is an important factor for mortality and morbidity (3, 4). These arrhythmias often occur as ventricular and atrial fibrillation (5, 6). Atrial fibrillation (AF) is the highest incidence of arrhythmias after CABG surgery (7). Arrhythmia has a direct relationship with the onset of ischemic time and reperfusion injury. Therefore, cardioplegic solution is used to prevent myocardial damage and develop its protection, and choosing a cardioplegic solution is important in this regard (3, 8). The cardioplegic solution protects the myocardium against ischemia and events during reperfusion (9). This solution contains different electrolytes and antiarrhythmic contents to stop the heart during distal anastomosis and protects the structure of the myocardium during the reperfusion period and its function after ischemia (10, 11). Several additives, such as beta-blockers, and calcium blockers can be used to prevent arrhythmia after aortic de-clamp (12). Lidocaine and procaine hydrochloride agents are two of the additives to cardioplegic solution that by decreasing the amount of extracellular sodium, cause cardiac arrest by removing substrate from the free sodium necessary for the potential for action and are supported by a small amount of extracellular charge of potassium, and with the stabilization of the cell membrane, it stablishes the heart rhythm after ischemic time (9, 10). In this study, the effects of these two drugs in the cardioplegic solution on post aortic de-clamp arrhythmia were assessed and compared so that using a suitable cardioplegia could reduce the occurrence risk of arrhythmia after aortic de-clamp and, consequently, the complications that follow it will be prevented.
2. Methods
This is a randomized clinical trial that was conducted in the operating room of the Rajaie cardiovascular, Medical and research center after obtaining permission from the ethics committee (rhc.ac.ir.rec.1396.41) from October 2016 to March 2017. Approved proposal was registered at the Iranian center for clinical trials (IRCT) (IRCT20171002036506N2).
Inclusion criteria was patients who were candidates for isolated elective coronary artery bypass graft surgery and consensus form was obtained. Exclusion criteria included emergent, re-do or concomitant valve surgeries, history of cardiac arrhythmia, left ventricle ejection fraction of less than 30%, severe aortic insufficiency, end stage renal disease, history of hypersensitivity to lidocaine. Also patients who needed re-exploration for post-operative bleeding control were excluded from this study. Demographic data and underlying diseases, clinical information and arterial blood gas, post-ischemic time events (Arrhythmia, need for DC shock, electrolyte imbalance), and early post-operative data including the occurrence of arrhythmia, need for inotrope and anti-arrhythmic drugs, ECG and echocardiographic findings. Based on the previous study (11), considering 90% strength of the study and the significance level of 0.05, the number of patients required for study in each group was estimated to be 50.
Statistical analysis was performed on raw data using SPSS 22 software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). One-sample Kolmogorov Smirnov test and Student’s t test (Mann Whitney U), Chi-square test and Fisher’s exact test were used. Multivariate analysis was performed using logistic regression models. P value less than 0.05 was considered statistically significant.
Balanced block randomization was used to assign patients to the two study groups of 1, the group of cardioplegic solution containing procaine hydrochloride (Shahid Ghazi Pharmaceutical Co. Tabriz, Iran) and 2, the group of cardioplegic solution with lidocaine additive. The cardioplegic solution was prepared according to the conditions and requirements under the supervision of the center’s pharmacist and the pharmaceutical department, and then the drugs were numbered and blinded by sealed envelopes method. Therefore none of the perfusionist, nurses, surgeons and researcher knew about the type of cardioplegic solution used. To evaluate the effect of these two drugs, the cardioplegic solution was prepared in such a way that, apart from the two antiarrhythmic drugs, there was no difference in electrolytes, osmolarity and their formulation. The composition and formulation of the cardioplegic solution are similar to the solution of ST.Thomas1, which is made and consumed in European countries by Martindale pharmaceuticals company with the registration number pl01883 / 0012. The latest drug review date was in February 2015, and is also being used in Australia under the name of DBL sterile cardioplegia concentrate (https://www.medicines.org.UK) (13). The Iranian sample is a cardioplegic solution prepared by Shahid Ghazi company, which in terms of composition and content is similar to the solution produced in Australia. This solution is in a volume of 50 mL; diluted in 1 liter of the ringer serum at 4°C. Each liter of diluted solution contains 44 milliequivalent (mEq) of potassium, 64 mEq of magnesium, 147 mEq of sodium and 4.5 mEq of calcium, and one millimole of procaine hydrochloride with osmolarity of 300 to 320 and a pH of about 7.4 to 7.6. This solution was used as the cardioplegic solution containing procaine hydrochloride and the other solution was prepared with the same formulation, osmolarity and PH, and only lidocaine 2% has been replaced instead of Procaine hydrochloride containing 5 cc (equivalent to 100 mg). Blood cardioplegia was used and electrolytes, especially potassium, were within the normal range and were given 1 to 1 cardioplegia and blood. Cardioplegic solutions were administered via aortic root at a rate of 300 cc per minute, for three minutes until a complete cardiac arrest was achieved. The method of induction and maintenance of anesthesia, surgical procedures, perfusion and the pump device, the model of the oxygenator, the method of priming, heparinization, the infusion method of cardioplegic solution (antegrade in the root of the aorta) and restoration of the effect of heparin by protamine were considered to be administered similarly for all patients. All CABGs were performed by the same surgical team. Demographic data including age, sex, weight, height, BSA and also troponin levels and preoperative ejection fraction, history of hypertension, thyroid, smoking, history of MI and angioplasty and beta blocker drugs were recorded. After aortic de-clamp, if the rhythm returned in 3 - 5 minutes spontaneously, it would be recorded as a self-reversal rhythm, otherwise lidocaine and magnesium are injected (potassium values are checked to make sure they are in the normal range). Variables such as hemodynamic parameters and arterial blood gas parameters were measured at the entry to the operating room, during the cross-clamp time, after aortic de-clamp, at the end of bypass pump, at the entrance to the ICU and after the extraction of the tracheal tube in the ICU. The volume and frequency of cardioplegic solution administration, the rate of spontaneous return to baseline heart rhythm, the need for electrical cardioversion after aortic de-clamp, the frequency of arrhythmia after declamping, the duration of arrhythmia, the need for lidocaine, magnesium and potassium were all recorded by perfusionist in the registered questionnaires. Also early postoperative data including the need for inotrope or pacemaker, or atrial and ventricular dysrhythmia, new ST-T changes in ECG, serum troponin level, mechanical ventilation time, ICU stay and post-operative echocardiographic findings have been collected.
3. Results
The two groups were similar in terms of demographic data and underlying disease, basic clinical data and during operation, therefore both study groups were well matched (Table 1).
| Variables | PHC Group | Lidocaine Group | P Value |
|---|---|---|---|
| Age, y | 62 ± 9.0 | 65 ± 4.9 | 0.08 |
| Gender, M/F | 31/19 | 30/20 | 0.83 |
| Weight, kg | 74 ± 9.9 | 74 ± 7.2 | 0.97 |
| BSA | 1.8 ± 0.17 | 1.8 ± 0.12 | 0.82 |
| Pre-op LVEF, mean % | 46 ± 6.4 | 48 ± 4.1 | 0.10 |
| Graft No. | 169 | 168 | 0.12 |
| Pre-op troponin | 1.6 (0.7 - 3.5) | 1 (0.6 - 3.6) | 0.95 |
| MAP, mmHg | 72 ± 15.5 | 77 ± 8.6 | 0.07 |
| HR | 77 ± 7.6 | 74 ± 5.3 | 0.10 |
| NSR | 50 | 50 | > 0.99 |
Patient Characteristics in Each Study Groups
As effective variables during the operation, the duration of the aortic valve cross clamp, potassium, magnesium, the patient’s body temperature during and after the aortic cross clamp, the volume and frequency of cardioplegia infusion, the number of grafts and the use of internal mammary artery were investigated (Table 2).
| Variable | Procaine | Lidocaine | P Value |
|---|---|---|---|
| AOX time, min | 41.5 ± 14.5 | 41.2 ± 9.5 | 0.89 |
| CPB time, min | 67.8 ± 10.2 | 69.2 ± 8.5 | 0.78 |
| K (during AOX) | 4.06 ± 0.6 | 3.8 ± 0.28 | 0.03 |
| Mg (during AOX) | 2.1 ± 0.3 | 2.0 ± 0.2 | 0.11 |
| BT (during AOX), °C | 34.4 ± 0.6 | 34.4 ± 0.3 | 0.25 |
| K (after AOX) | 4.8 ± 0.75 | 4.6 ± 0.57 | 0.17 |
| Mg (after AOX) | 2.0 ± 0.26 | 2.1 ± 0.57 | 0.22 |
| BT (after AOX), °C | 36.3 ± 0.5 | 36.2 ± 0.2 | 0.63 |
| Cardioplegia volume, CC | 1406 ± 312 | 1130 ± 267 | 0.63 |
| Cardioplegia frequency | 0.59 | ||
| 1 | 25 (50) | 27 (54) | |
| 2 | 18 (36) | 18 (36) | |
| 3 | 6 (12) | 5 (10) | |
| 4 | 1 (2) | 0 (0) | |
| Graft number | 0.12 | ||
| 1 | 0 (0) | 0 (0) | |
| 2 | 1 (2) | 0 (0) | |
| 3 | 29 (58) | 38 (76) | |
| 4 | 20 (40) | 12 (24) | |
| Lima usage | 45 (90) | 47 (94) | 0.46 |
| Graft No. | 169 | 168 | 0.12 |
Comparison of the Values of Effective Variables During Surgery in the Two Studied Groups (N = 50)a
According to the raw data available in our Table, although the difference between the two groups regarding the potassium level were statistically significant, since both values were in normal ranges, this difference cannot be clinically important.
Also despite the fact that the frequency of cardioplegia infusion was statistically more in procaine amid group, cardioplegic solution volume infusion seems to be similar in both groups.
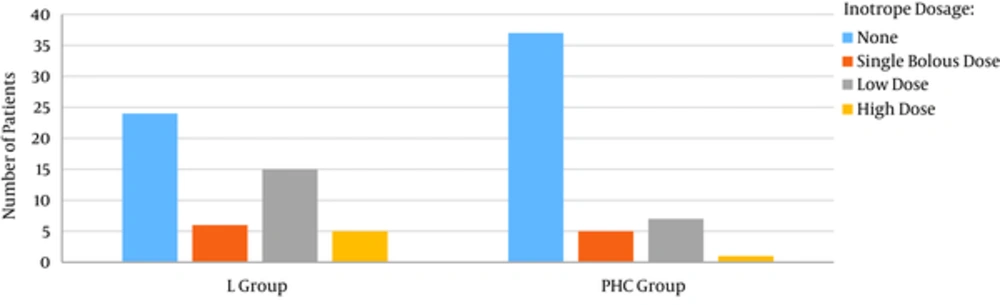
Generally the need for inotrope for cardiopulmonary bypass weaning was significantly higher in lidocaine group. The need for high dose inotrope (More than 0.1 mcg/kg/min) was also higher in lidocaine group (10% vs. 2%) (Figure 1).
| Variable | Procaine | Lidocaine | P Value |
|---|---|---|---|
| Spontaneous sinus rhythm | 32 (64) | 21 (42) | 0.02 |
| Lidocaine + Mgb | 18 (36) | 29 (58) | 0.02 |
| Atrial Arrhythmias | 0.58 | ||
| PSVT | 7 (14) | 11 (22) | |
| AF | 1 (2) | 1 (2) | |
| Ventricular arrhythmias | 0.10 | ||
| VT | 1 (2) | 6 (12) | |
| VF | 2 (4) | 5 (10) | |
| Transient PVC | 2 (4) | 1 (2) | |
| Defibrillator | 3 (6) | 6 (12) | 0.29 |
| Pacemaker | 0.40 | ||
| Transit pacemaker | 2 (4) | 4 (8) | 0.68 |
| Permanent pacemaker | 0 (0) | 0 (0) | < 0.99 |
| Arrhythmia time | 6.2 ± 4.59 | 6.5 ± 5.79 | 0.76 |
| New ST-T change | 1 (2) | 0 (0) | > 0.99 |
Comparison of Indicators Related to Arrhythmia and Return of Heart Rhythm in the Study Groups (N = 50)a
A total of 64% of the patients in the procaine hydrochloride group and 48% of the patients in the lidocaine group experienced the spontaneous return of the heart rhythm at the time of the aortic valve opening. The amounts of lidocaine and magnesium required for returning to the normal sinus rhythm were comparatively lower in the procaine hydrochloride group (36% vs. 58%).
Inotropic drugs have been studied in three types in the two groups, which in general were lower in procaine hydrochloride group (P = 0.04). However, there was no statistically significant difference between the two groups in the incidence of atrial arrhythmias that were analyzed for both AF and PSVT and also for ventricular arrhythmias that were compared in three modes of VF, VT and Transient PVC.
In the procaine hydrochloride group, 3 patients required defibrillator due to arrhythmia after the opening of the aortic valve, of which 2 patients needed a pacemaker for having a sinus rhythm. Although, at the end of the operation, the heart had a sinus rhythm and the pacemaker has been stopped. However, in the lidocaine group, 6 patients needed a defibrillator, of which 4 of them needed a pacemaker afterwards. Among these four patients, two of them had a sinus rhythm at the end of the operation and the pacemaker has been stopped, and the two others were transferred to intensive care unit with a heart pacemaker.
In the intensive care unit, only one patient in the procaine hydrochloride group suffered from arrhythmia, where defibrillator and pacemaker were used, which eventually returned to sinus rhythm after a few hours and the heart pacemaker was stopped. In the two patients from lidocaine group, which had been transferred from the operating room with a pacemaker, the heart rhythm returned to normal after a few hours and the pacemaker was stopped. There was no statistical difference in the results of the study between the two groups in intensive care unit.
In Tables 4 and 5, the effect of each of the variables of the studied groups including age, sex, history of myocardial infarction, potassium and magnesium, the opening time of the aortic valve and the duration of the aortic valve cross clamp have separately and with the removal of the effect of other confounding factors on the spontaneous return of heart rhythm and the need for inotropic drugs been investigated.
| Variable | Coefficient | SE | P Value | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Study groups | 1.03 | 0.44 | 0.02 | 2.81 | 1.17 | 6.76 |
| Age | 0.01 | 0.03 | 0.58 | 1.01 | 0.95 | 1.08 |
| Gender | -0.17 | 0.44 | 0.68 | 0.83 | 0.35 | 1.98 |
| History of MI | -1.06 | 0.55 | 0.05 | 0.34 | 0.11 | 1.02 |
| K (after AOX) | -0.24 | 0.32 | 0.44 | 0.78 | 0.41 | 1.48 |
| Mg (after AOX) | -0.87 | 0.93 | 0.34 | 0.41 | 0.06 | 2.60 |
| AOX time | -0.03 | 0.01 | 0.08 | 0.96 | 0.93 | 1.00 |
Adjusted Correlation Between Spontaneous Sinus Rhythm and Each of the Variables
| Variable | Coefficient | S.E. | P Value | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Study groups | -1.18 | 0.46 | 0.01 | 0.30 | 0.12 | 0.75 |
| Age | 0.05 | 0.03 | 0.18 | 1.05 | 0.97 | 1.13 |
| Gender | 0.41 | 0.45 | 0.36 | 1.51 | 0.62 | 3.70 |
| History of MI | 0.86 | 0.54 | 0.11 | 2.37 | 0.81 | 6.93 |
| K (after AOX) | 0.18 | 0.34 | 0.58 | 1.20 | 0.61 | 2.36 |
| Mg (after AOX) | -0.03 | 0.96 | 0.97 | 0.96 | 0.14 | 6.40 |
| AOX time | 0.02 | 0.01 | 0.25 | 1.02 | 0.98 | 1.06 |
Adjusted Correlation Between Inotrope and Each Variable
These Tables show that the studied groups and each of the cardioplegic solutions affect the spontaneous return of heart rhythm (P = 0.02) and the need for inotropic use (P = 0.01). Also, the history of MI is effective on the spontaneous return of the heart rhythm (P = 0.05).
4. Discussion and Conclusion
Cardiac arrhythmias is one of the most common complications after open heart surgery, which is an important factor for mortality and morbidity (3, 4). The incidence of post-operative ventricular arrhythmias may range from 1.8% - 13% (14). Ventricular fibrillation status and attempts for treatment by DC shock may injure the myocardium during reperfusion (15). Although there several anti-arrhythmic agents can be used to prevent post-operative arrhythmia after aortic declamping, there are reports that show amiodarone may be more effective in preventing post-operative arrhythmias (16). Lidocaine affects the sodium channels and decreases the late depolarization and by increasing the diastolic electric current shock works an anti-arrhythmic agent (17). Procaine hydrochloride is also a local anesthetic agent that may have antiarrhythmic role and has a similar action mechanism with Lidocaine. These arrhythmias often occur as ventricular and atrial fibrillation (5, 6). Atrial fibrillation (AF) is the highest incidence of arrhythmias after CABG surgery (7). Arrhythmia has a direct relationship with the onset of ischemic time and reperfusion injury. Therefore, cardioplegic solution is used to prevent myocardial damage and develop its protection, and choosing a cardioplegic solution is important in this regard (3, 8). The cardioplegic solution protects the myocardium against ischemia and events during reperfusion (9). Lidocaine and procaine hydrochloride agents are two of the additives to cardioplegic solution that by decreasing the amount of extracellular sodium, cause cardiac arrest by removing substrate from the free sodium necessary for the potential for action and are supported by a small amount of extracellular charge of potassium, and with the stabilization of the cell membrane, it stablishes the heart rhythm after ischemic time (9, 10).
In the present study, there is a significant difference between two groups in the comparison of spontaneous sinus rhythm return after declamping and spontaneous return was higher in procaine hydrochloride group. The amount of lidocaine and magnesium and inotropic drugs required for sinus rhythm was lower in procaine hydrochloride group and the results are similar to those of Sellevold (10) study results where two cardioplegic solutions, one containing 1 mM Procaine hydrochloride have been compared to a cardioplegic solution containing 0.9% normal sodium in the Lidocaine group, and the incidence of VF after aortic declamping and the effect of procaine hydrochloride on the reduction of ventricular fibrillation after declamping have been investigated. In our study, the percentage of atrial arrhythmias in the two groups was 16% - 24% and the incidence of ventricular arrhythmias between the two groups was 10% - 24% and there was no significant difference between the two groups in regards to the occurrence of atrial and ventricular arrhythmias. In a similar study done by Peretto (7) in 2012, AF is referred to as usual and common early postoperative premature arrhythmia, with a 15 to 40% chance of occurrence in coronary procedures. Overall, the present study shows that the amount of required cardioplegia was higher for the solution containing procaine hydrochloride and lower for the solution containing Lidocaine. Perhaps the amount of volume required for a solution containing procaine hydrochloride may be related to its steric characteristics. Procaine hydrochloride is a local anesthetic agent and can easily hydrolyze in the body loses its effect (18). In two similar studies published by Mishra (19) and Buel (20) in 2016, the aortic valve clamp time and the required cardioplegic dose were relatively lower in the group using the del Nido cardioplegic solution (containing lidocaine) and EF after surgery was better than St. Thomas group.
In summary, the volume of cardioplegic solution and the number of repeat doses of the solutions was more in procaine hydrochloride group. The spontaneous sinus rhythm return was more in solutions containing procaine hydrochloride and there was lees need for Lidocaine and Magnesium to get back to the sinus rhythm. However, there was no significant difference in the two groups regarding the need for defibrillator and pacemaker. Therefore, according to our study procaine hydrochloride does not seem to play a crucial role in reducing post-operative cardiac arrhythmias. Obviously more researches with higher number of study groups and other complex cardiac surgeries are required to achieve more reliable conclusions.
4.1. Study Limitations
This study was performed on limited number of selected non-complex CABG patients in a single center.