1. Background
A thrombosis is one of the most important problems in cardiovascular surgery patients, and active platelets play a significant role in this process. Larger platelets are metabolically and enzymatically more active than smaller ones (1), and they contain more granules and vasoactive mediators, such as thromboxane A2 and serotonin (2, 3). Previously, it has been reported that a high mean platelet volume (MPV) is associated with a number of cardiovascular diseases (4, 5). For example, high MPVs have been shown to be independent predictors of ischemic events, recurrent myocardial infarction (MI), or death from coronary artery disease (6-8). Additionally, Tavil et al. (9) demonstrated that the platelet volume plays an important role in late saphenous vein graft disease. Furthermore, higher MPVs have been observed in patients with diabetes mellitus (DM) (10), hypertension (HT) (11), hypercholesterolemia (12), and smoking (13), which suggests a common mechanism by which these factors may increase the risk of cardiovascular disease. Thus, a high MPV has been seen as a disadvantage in cardiovascular patients via the mechanism of graft thrombosis.
Although high MPVs have been related to increased morbidity and mortality via thrombosis, Magri et al. (14) observed that a lower MPV is a significant predictor of bleeding in subjects undergoing transfemoral transcatheter aortic valve implantations. Therefore, the aim of this study was to investigate the relationship between postoperative hemorrhagic pleural effusion (PE) and the MPV during the early period after coronary artery bypass graft (CABG) surgery.
2. Methods
2.1. Study Design and Population
Patients who underwent isolated CABGs between January 2012 and January 2013 were included in this retrospective case-control study. At our facility, we perform control chest X-rays two weeks after surgery (about one week after discharge) in our clinical routine practice. If the pleural fluid level covered more than 25% of the hemithorax in the control chest X-ray, it was determined to be major PE, as in the study by Labidi et al. (15). Consequently, 77 patients who had major PE in their control chest X-rays were included in the study group. Ninety-two patients with pleural fluid levels covering less than 25% of the hemithorax in the control chest X-ray were selected randomly, and they were included in the control group. A statistical comparison was performed between the PE and non-PE groups in terms of the clinical parameters (Table 1), laboratory parameter measurements upon admission and upon discharge (Table 2), operative parameters (Table 3), and postoperative parameters (Table 4).
| Clinical Parameters | Pleural Effusion Group (N = 71)a | No Pleural Effusion Group (N = 85)a | P Value |
|---|---|---|---|
| Age, y | 60.3 ± 11.8 | 57.9 ± 11.3 | 0.201 |
| Gender | |||
| Male | 48 (67.6) | 70 (82.4) | 0.033b |
| Female | 23 (32.4) | 15 (17.6) | |
| BMI, kg/m2 | 28.1 ± 4.3 | 28.5 ± 6.0 | 0.576 |
| DM | 26 (36.6) | 23 (27.1) | 0.200 |
| HT | 54 (76.1) | 60 (70.6) | 0.443 |
| COPD | 16 (22.5) | 16 (18.8) | 0.568 |
| PAD | 5 (7) | 9 (10.6) | 0.577 |
| Stroke | 0 | 0 | 1 |
| Smoking, pocket/y | 19.1 ± 19.6 | 18.2 ± 18.5 | 0.753 |
| Emergency | 10 (14.1) | 6 (7.1) | 0.150 |
| EF, % | 52.4 ± 9.7 | 54.2 ± 9.0 | 0.234 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; EF, ejection fraction; HT, hypertension; PAD, peripheric arterial disease; SD, standard deviation.
aValues are expressed as mean ± SD or No. (%).
bStatistically significant.
| Laboratory Parameters | Pleural Effusion Group (N = 71)a | No Pleural Effusion Group (N = 85)a | P Value |
|---|---|---|---|
| Urea, mg/dL | |||
| Preoperative | 18.6 ± 9.4 | 17.7 ± 9.1 | 0.524 |
| Discharging | 18.3 ± 9.4 | 19.7 ± 10.5 | 0.362 |
| Creatinine, mg/dL | |||
| Preoperative | 0.96 ± 0.28 | 1.0 ± 0.74 | 0.579 |
| Discharging | 1.0 ± 0.4 | 1.0 ± 0.6 | 0.911 |
| Hemoglobin, g/dL | |||
| Preoperative | 15.0 ± 2.0 | 15.2 ± 2.2 | 0.494 |
| Discharging | 10.3 ± 1.0 | 10.2 ± 1.0 | 0.312 |
| Hematocrit, % | |||
| Preoperative | 45.1 ± 6.0 | 44.9 ± 6.1 | 0.780 |
| Discharging | 32.9 ± 2.7 | 32.8 ± 2.6 | 0.805 |
| Platelet count, ×109/L | |||
| Preoperative | 267.5 ± 80.5 | 261.3 ± 105.6 | 0.687 |
| Discharging | 224.6 ± 58.8 | 226.2 ± 53.0 | 0.860 |
| MPV, fL | |||
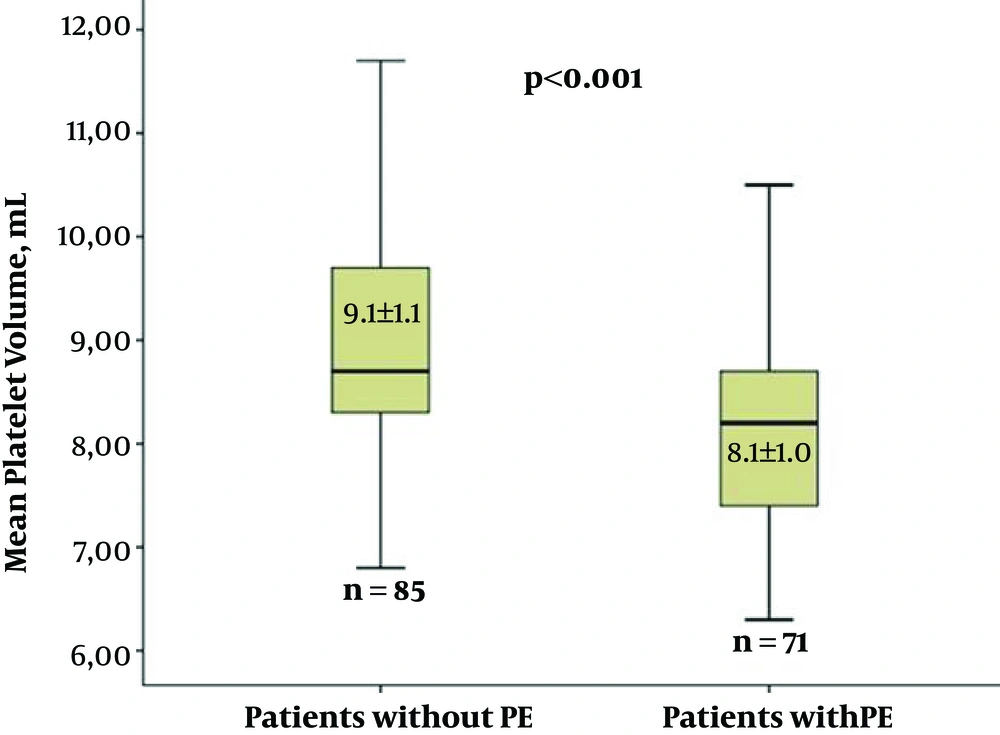
| Preoperative | 8.1 ± 1.0 | 9.1 ± 1.1 | < 0.001b |
| Discharging | 8.5 ± 0.9 | 9.2 ± 0.9 | < 0.001b |
| Total cholesterol, mg/dL | 198.2 ± 44.0 | 188.3 ± 42.6 | 0.156 |
| LDL, mg/dL | 114.1 ± 37.3 | 110.3 ± 33.5 | 0.497 |
| HDL, mg/dL | 46.8 ± 11.1 | 50.7 ± 14.1 | 0.059 |
| Trigliserid, mg/dL | 187.3 ± 77.3 | 176.8 ± 75.7 | 0.395 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MPV, mean platelet volume; SD: standard deviation.
aValues are expressed as mean ± SD.
bStatistically significant.
Abbreviations: ACCT, aortic cross clamp time; CPBT, cardiopulmonary bypass time; LIMA, left internal mammariyal artery; SD, standart deviation.
aValues are expressed as mean ± SD or No. (%).
| Postoperative Parameters | Pleural Effusion Group (N = 71)a | No Pleural Effusion Group (N = 85)a | P Value |
|---|---|---|---|
| Length of intubated time, h | 12.0 ± 13.9 | 10.3 ± 6.1 | 0.296 |
| Length of stay in ICU, d | 2.9 ± 8.4 | 1.3 ± 0.8 | 0.109 |
| Length of stay in hospital, d | 5.9 ± 1.4 | 5.9 ± 1.7 | 0.821 |
| Total drenage, mL | 831 ± 606.2 | 893 ± 659 | 0.545 |
| Diuretic at discharge | 11 (15.5) | 9 (10.6) | 0.362 |
| Warfarin at discharge | 7 (9.9) | 8 (9.4) | 0.925 |
Abbreviation: ICU, intensive care unit.
aValues are expressed as mean ± SD or No. (%).
Bleeding revision, an ejection fraction (EF) of less than 35%, a beating heart CABG, redo surgery, absolute serous fluid in the paracentesis, and malignancies were accepted as exclusionary criteria. Therefore, 6 patients who underwent beating heart surgery, 5 patients with EFs of less than 35%, and 2 patients with known malignancies were excluded from the study. As a result, the PE group and the non-PE group populations were composed of 71 and 85 patients, respectively. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared (kg/m2). Since the height is commonly measured in centimeters, the value was divided by 100 in order to obtain the height in meters. HT was defined as a blood pressure of 140/90 mmHg or greater in three measurements. DM was defined as a fasting blood glucose of > 126 mg/dL in two measurements preoperatively, or if the patient was treated with insulin or an oral hypoglycemic medication. Chronic obstructive pulmonary disease was defined as a forced expiratory volume in one second/forced vital capacity of less than 70%, or if the patient was under bronchodilator treatment. The MPV is measured in routine hemogram (complete blood count) examinations, so more advanced examinations were not required to obtain the MPV.
All the patients were operated on by the same surgical team. A median sternotomy was the preferred surgical approach in all unnecessary patients. In this cohort, all the patients underwent cardiopulmonary bypasses in the standard fashion using mild hypothermia (32 - 34ºC). The chest drainage tube was inserted via the lateral side of chest.
This study was conducted in accordance with the guidelines of the Helsinki Declaration, and it was not supported by any company. In addition, this study was approved by the ethics committee of our hospital. We obtained each patient’s written informed consent to be included in this study. The authors received no financial support for the research and/or authorship of this article.
2.2. Statistical Analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences, version 16.0 (SPSS Inc., Chicago, IL, USA). The continuous data were expressed as the mean ± standard deviation, while the categorical data were presented as percentages. The Chi-squared test was used to compare the categorical variables, while the Student’s t test or the Mann-Whitney U test were used to compare the parametric and nonparametric continuous variables, respectively. The normal distribution was assessed by the Kolmogorov-Smirnov test. A logistic regression analysis was performed to determine the postoperative PE predictors. A value of P < 0.05 was considered to be statistically significant.
3. Results
Overall, 156 patients were enrolled in this study, including 118 (75.6%) males and 38 (24.3%) females. The mean age of the patients was 58.9 ± 11 years old. Paracenteses were performed in those patients whose pleural fluid level covered over 25% of the hemithorax. A tube thoracostomy was performed in 35 of the 71 PE patients, while thoracenteses were performed in 23 of them. Thirteen of the patients were treated with diuretic medications only. Microscopic and biochemical analyses were not performed on the pleural fluid, but absolute serous fluid was not observed macroscopically in any of these patients.
The number of males was significantly higher (P = 0.033) in the non-PE group. However, there were no significant differences between the groups with and without PE in terms of the other demographic parameters (Table 1). Two separate laboratory parameter measurements (upon admission and upon discharge), including the hemoglobin, hematocrit, platelets, urea, creatinine, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglycerides were compared statistically, and there were no significant differences between the groups (Table 2). Additionally, the MPV levels upon admission and discharge were significantly higher (P < 0.001) in the non-PE group (Figure 1). During the surgery, the left internal mammary artery (LIMA) usage, graft count, degree of hypothermia, cardiopulmonary bypass time (CPBT), and aortic cross-clamp time (ACCT) were compared in terms of PE development, and there were no differences between the two groups (Table 3). During the postoperative period, the length of intubation time, length of stay in the intensive care unit (ICU), amount of total drainage, length of stay in the hospital, use of diuretics (Furosemide and/or Aldactazide), and use of warfarin postoperatively were statistically similar between the two groups (Table 4).
Univariate logistic regression analyses were performed to determine the postoperative PE predictors. The number of males and the MPV were statistically significant high in the non-PE group [odds ratio (OR) = 2.236, 95% confidence interval (CI) = 1.059 - 4.720, P = 0.035 and OR = 0.371, 95%CI = 0.247 - 0.557, P < 0.001, respectively] (Table 5). The multivariate logistic regression analysis showed that an elevated MPV was an independent predictor of decreased postoperative PE (OR = 0.375, 95%CI = 0.248 - 0.567, P < 0.001) (Table 6 and Figure 1).
| OR | 95%CI for OR | P Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, y | 1.018 | 0.990 | 1.047 | 0.201 |
| Gender | 2.236 | 1.059 | 4.720 | 0.035 |
| Warfarin at discharge | 1.053 | 0.362 | 3.060 | 0.925 |
| HT | 1.324 | 0.646 | 2.712 | 0.444 |
| DM | 1.557 | 0.789 | 3.074 | 0.201 |
| BMI | 0.983 | 0.924 | 1.045 | 0.575 |
| Total cholesterol, mg/dL | 1.005 | 0.998 | 1.013 | 0.157 |
| Hemoglobin, g/L | 0.949 | 0.817 | 1.102 | 0.492 |
| Emergency | 2.158 | 0.743 | 6.267 | 0.157 |
| ACCT, min | 1.016 | 0.999 | 1.033 | 0.072 |
| MPV, fL | 0.371 | 0.247 | 0.557 | < 0.001a |
Abbreviations: ACCT, aortic cross clamp time; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HT, hypertension; MPV, mean platelet volume; OR, odds ratio.
aStatistically significant.
| OR | 95%CI for OR | P Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, y | 1.011 | 0.979 | 1.044 | 0.504 |
| Gender | 2.094 | 0.867 | 5.059 | 0.101 |
| Warfarin at discharge | 1.919 | 0.552 | 6.680 | 0.305 |
| Emergency | 1.744 | 0.509 | 5.971 | 0.376 |
| ACCT, min | 1.014 | 0.994 | 1.034 | 0.179 |
| MPV, fL | 0.375 | 0.248 | 0.567 | < 0.001a |
Abbreviations: ACCT, aortic cross clamp time; CI, confidence interval; MPV, mean platelet volume; OR, odds ratio.
aStatistically significant.
4. Discussion
The primary outcomes of this study suggest that the MPV and the number of males were significantly lower in the postoperative PE group. Furthermore, according to the multivariate analyses, we found that only a lower MPV was an independent predictor of postoperative PE.
PE is a significant complication in CABG patients, and it is associated with postoperative morbidity and extended hospital and ICU stays. PE develops in 41% to 87% of CABG patients during the early period, as identified in chest X-rays (16-19). Labidi et al. (15) demonstrated that a history of heart failure, peripheral arterial disease, or atrial fibrillation can play a significant role in the development of PE in a CABG patient. Anticoagulants (heparin, low-molecular-weight heparin, and warfarin), clopidogrel, anti-arrhythmic agents, and diuretic agents have also been associated with PE development, which may be partially explained by the underlying disease for which such medications are generally prescribed. In this study, all the patients were taking acetylsalicylic acid both preoperatively and postoperatively. Four (5.6%) and 5 (5.9%) patients were using clopidogrel in the PE and non-PE groups, respectively.
Hurlbut et al. (19) reported that during the sixth postoperative day following a CABG, the left PE incidence was higher in those patients whose internal mammary artery was harvested (47%) than in those patients in whom only the saphenous vein was harvested (84%). According to Sadikot et al. (20), early PE is usually hemorrhagic, and late effusion is usually nonhemorrhagic. Moreover, Labidi et al. (15) analyzed the pleural fluid, and they observed that the numbers of erythrocytes, lymphocytes, and neutrophils and the pleural fluid lactate dehydrogenase (LDH)/serum LDH ratio were higher in the patients in which PE developed during the first 15 days than in those in which PE developed during the second 15 days. Thus, the pleural fluid exhibited hemorrhagic characteristics during the first 15 days.
Biochemical and cellular analyses were not performed in the pleural fluid in our study. Although every effusion fluid sample obtained via thoracentesis was serohemorrhagic, it may be necessary to evaluate the PE etiopathogenesis. Hemorrhagic diathesis, such as platelet dysfunction, warfarin, or acetylsalicylic acid usage, may be a factor in the development of hemorrhagic PE in patients with CABGs. We observed that the previously explained parameters, such as warfarin, clopidogrel and diuretic usage, peripheral artery disease, and LIMA harvesting, exhibited no effects on PE development. A low MPV only significantly affected PE development based on the multivariate analysis in this study.
The MPV shows platelet activation in that larger platelets are metabolically and enzymatically more active in terms of thrombotic processes than smaller ones (21). Therefore, larger platelets have more alpha-granules, including clotting mediators like factor V, factor VIII, fibrinogen, fibronectin, platelet-derived growth factor, and chemotactic agents (22). It is known that increased platelet activation and aggregation are closely related to cardiovascular complications (23). Numerous studies have shown that large platelets are predictors of thrombotic events in coronary and peripheral arterial disorders (24). For example, Dogan et al. (25) demonstrated that patients with non-ST-segment elevation MIs with high admission MPVs (≥ 9.9 fL) had more frequent major cardiac events, such as cardiac death, MI, recurrent angina, or hospitalization (39% vs. 26%), when compared to those with lower MPVs (< 9.9 fL). Contrarily, a higher MPV may be an advantage, leading to less bleeding than a lower MPV in patients with percutaneous transfemoral transcatheter aortic valve implantations. In addition, the major role of platelets was evaluated in bleeding complications after cardiac surgery by Mahla et al. (23). Similarly, we observed that a higher MPV led to a lesser chance of a hemorrhagic PE than a lower MPV in the CABG patients in our study. Despite all these factors, the pathophysiological mechanism of the relationship between the MPV and hemorrhagic PE is not clear in the literature. Moreover, the current study is a clinical study, and the mechanism is still not fully understood. Further studies are required to investigate and clarify these mechanisms.
There is insufficient evidence of the role that a thrombocyte transfusion plays in increasing the MPV in the literature. Gambling with the risks of a thrombocyte transfusion may not to be necessary for the sake of increasing the MPV.
Although the multivariate logistic regression analysis showed that there was no effect of gender on PE, gender did affect PE development in the univariate analysis in this study. PE was seen in a smaller number of male patients than female patients. Although the cause of this situation is not clear, Labidi et al. (15) demonstrated the same result, and they attributed this to the smaller body surface area and more intravascular volume in the male patients.
The results of this study showed that those patients with higher MPVs had a higher thrombotic potential than the patients with lower MPVs. Therefore, these patients had a greater cardiovascular risk. However, we demonstrated that the patients with higher MPVs had a lesser risk of hemorrhagic PE than the patients with lower MPVs during the postoperative period.
4.1. Study Limitations
This study has several limitations. First, it was a single-center retrospective study with a relatively small sample size. A longer follow-up period may be necessary to evaluate the long-term effects of the MPV on PE. Although a pleural fluid analysis was not performed, it may be necessary to evaluate the PE etiopathogenesis. Finally, the causes of PE in the patients with other cardiac surgeries were unknown in this study. Moreover, the study findings may be seem interesting, but have no significant clinical importance and may not change the routine clinical practice and it may be as an incidental findings.
4.2. Conclusion
Almost all the previous cardiovascular studies related to the MPV showed that a higher MPV was a poor prognostic indicator for cardiovascular outcomes. Contrarily, in the present study, we demonstrated that an increased MPV was independently associated with a lower incidence of PE in patients with CABGs during the early postoperative period. As a simple and widely available diagnostic test, the MPV can help predict PE development in CABG patients postoperatively.