1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, with a high prevalence increasing with ageing. In patients undergoing cardiac surgery, AF has been associated with enhanced perioperative complications and worse late survival (1). AF presents in approximately 60% - 80% of patients undergoing mitral valve (MV) surgery. Pharmacological management for AF has been associated with some limitations and a low success rate about 50% of cases (2), leading to the development of interventional procedures, including catheter ablation and surgeries (3).
The Cox-Maze procedure (CMP) is the gold standard procedure used in the surgical treatment of AF (4). On the other hand, experimental and clinical studies have consistently shown that focal discharges from the pulmonary veins are implicated in the initiation and perpetuation of AF (5). Therefore, this finding opened the door for catheter ablation of AF and pulmonary vein isolation (PVI) (6, 7). The CMP-III is accompanied by technical complexity and invasiveness; hence, it is replaced by a simplified procedure involving ablation lines, named CMP-IV (8). The latter provides surgeons with the option to implement a combination of bipolar radiofrequency (RF) ablation or cryoablation (9).
Rheumatic heart disease (RHD) is the most common cause of AF in developing countries and more than 80% of preoperative chronic AF remains after valvular surgery alone (4). The major cardiac and surgical societies have recommended that concomitant surgical approaches for AF should be performed in all cases when feasible (10). Herein, we aimed to evaluate the clinical outcomes of the N2O-based surgical cryo-PVI in RHD patients with long-standing persistent AF undergoing MV surgeries with or without concomitant surgeries and to compare these findings with those of the biatrial cryoablative CMP-IV.
2. Methods
2.1. Study Protocol and Population
In a prospective observational study from February 2010 to November 2012, a total of 65 consecutive patients with rheumatic MV disease associated with long-standing persistent AF, defined as AF lasting more than 6 months who were scheduled to undergo MV replacement (MVR) or MV repair with or without additional cardiac operations, were enrolled into the study. The cryoablative operation was performed either as biatrial CMP-IV in 25 patients (CMP-IV group) or as surgical cryo-PVI in 40 patients (PVI group). This study was approved by the local ethics committee in Rajaie cardiovascular medical and research center and written informed consents were also obtained from the patients.
2.2. Surgical Techniques
All procedures were performed by a single surgeon. The biatrial CMP-IV was performed with a wide-angled cryoprobe (Danesh® Medical Engineering Co., Tehran, Iran). The N2O-based cryoablation device is a newly designed tool that is approved by the Iran ministry of health; therefore, the patients were informed. The probes were cooled down at least to -60°C. In order to have a maximum tissue penetration during cryoablation, 2 minutes of tissue contact was implemented.
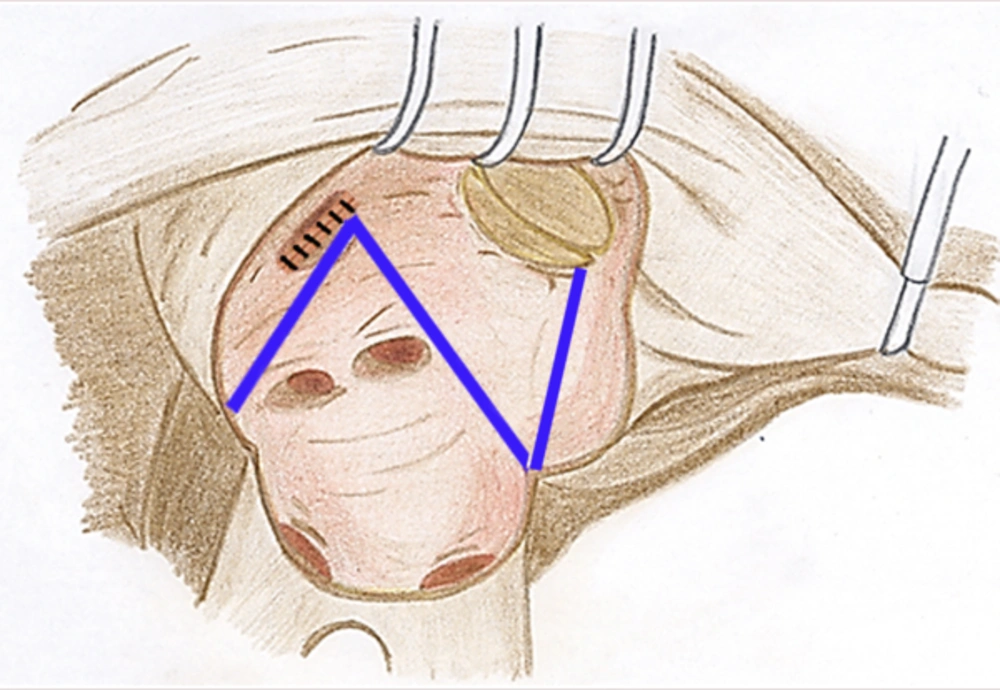
All patients had long-standing RHD with persistent AF. The MV operations were done after the induction of general anesthesia through a classic midline sternotomy. Cardiopulmonary bypass was then established using ascending aorta and bicaval cannulations associated with applying moderate hypothermia (32°C). They underwent preoperative transesophageal echocardiography to confirm the absence of left atrial (LA) thrombus. In some cases, concomitant procedures including coronary artery bypass grafting or other valvular surgery were also carried out. All patients underwent closure of the left atrial appendage (LAA) to reduce the risks of stroke as well. In the PVI group, the cryosurgical ablation was performed as follows: 1) LA atriotomy incision was applied; 2) MV was evaluated for implementing either MVR or MV repair; 3) In the cases of MVR, MV was excised and then cryosurgical ablation performed and consequently a suitably sized prosthetic valve was replaced; 4) in the cases of MV repair, first the cryosurgical ablation was done and then MV was repaired. The cryoablative lines were drawn to encompass the PVs and an additional ablation line was drawn from the left surgical atriotomy to the posterior MV annulus (Figure 1). In the CMP-IV group, in addition to the above-mentioned lines, other right atrial and atrial septum cryoablation lines were applied as follows: 1) Right atrial (RA) appendage was excised and right atriotomy extended up to the mid part of the RA; 2) a line was placed on atrial septum; 3) a line was made between superior and inferior vena cavas; 4) a line was made from the excised RA appendage to the annulus of tricuspid valve (TV) away from atrioventricular conduction system; 5) after creating a small hole in anterior part of the RA adjacent to the posterior leaflet of TV, two other lines were made, including a line from the hole to the middle part of the first line between vena cavas and another extending from the hole to the TV annulus. Moreover, intraoperative transesophageal echocardiography was done for all patients. Two right ventricular and two RA epicardial pacing wires were placed in all patients for dual-chamber pacing when needed. There was no preference for selecting the ablation procedures in our cohort. After surgery, all patients received heparin continued by warfarin to reach an international normalized ratio (INR) of 2.5-3.5, and then went on warfarin regimen alone. No electrical or medical cardioversion were applied postoperatively to restore sinus rhythm.
2.3. Follow-Up
The patients’ follow-up assessment was applied in three time intervals, including in-hospital, 1-month, and late periods. After surgery, antiarrhythmic drugs were continued using propranolol (20 - 80 mg/day) or metoproterenol (25 - 100 mg/day). At each follow-up interval, the patients underwent physical examination, electrocardiogram evaluation, and Holter monitoring in patients with normal sinus rhythm. Echocardiographic evaluations were performed at each follow-up except for the late follow-up at which echocardiography was performed in patients with suspicion of prosthetic valve dysfunction.
2.4. Statistical Analysis
Continuous variables were reported as mean ± standard deviation or median (interquartile ranges); those were analyzed using t-test or Mann-Whitney U test as appropriate. Categorical variables were reported as number (percentage) and Chi-square test or Fisher Exact tests were used as appropriate. Receiver operating characteristic (ROC) curve was used for identifying the best cut-off point for LA size to detect the patients with AF at late follow-up. The Cox regression analysis was also conducted to identify the main predictors of late AF during follow-up period. All variables which have been found to be mainly associated with AF recurrence after AF surgery were used as covariates (11, 12). Two-sided P values were calculated. All statistical analyses were performed using SPSS statistical software, version 21.0 (IBM Corp).
3. Results
3.1. Baseline and Clinical Outcomes
Out of 65 patients, 25 patients underwent biatrial cryoablative CMP-IV and 40 patients underwent surgical cryo-PVI for long-standing persistent AF. Patients’ mean age was 49.6 ± 10.6 years and female sex (67.7%) was predominant in our cohort. MVR alone and MV repair alone were performed in 23 patients (35.4%) and 9 patients (13.8%), respectively. The most concomitant surgery with MVR was tricuspid valve (TV) repair (28% in the CMP-IV and 20% in the PVI groups, P = 0.456). Other characteristics are summarized in Table 1.
| Total (n = 65) | CMP IV (n = 25) | PVI (n = 40) | P Value | |
|---|---|---|---|---|
| Age, y | 49.6 ± 10.6 | 43.8 ± 9.9 | 53.2 ± 9.4 | < 0.001 |
| Gender, female | 44 (67.7) | 17 (68) | 27 (67.5) | 0.967 |
| AF duration, month | 12 (9 to 23) | 13 (9 to 32) | 11.5 (8.5 to 20.5) | 0.507 |
| Prior histories | ||||
| Diabetes mellitus | 5 (7.7) | 2 (8) | 3 (7.5) | 0.941 |
| Hypertension | 8 (12.3) | 4 (16) | 4 (10) | 0.474 |
| CVA | 7 (10.8) | 2 (8) | 5 (12.5) | 0.569 |
| CAD | 9 (13.8) | 3 (12) | 6 (15) | 0.733 |
| Digoxin consumption | 46 (70.8) | 18 (72) | 28 (70) | 0.863 |
| Echocardiographic features | ||||
| LA size, cm | 5.1 ± 1.1 | 5.2 ± 1.2 | 5 ± 1.1 | 0.472 |
| LA clot | 5 (7.7) | 3 (12) | 2 (5) | 0.303 |
| LVEF, % | 50 (45 to 55) | 50 (45 to 55) | 47 (45 to 50) | 0.131 |
| AV insufficiency | 25 (38.5) | 8 (32) | 17 (42.5) | 0.397 |
| AV stenosis | 4 (6.2) | 0 (0) | 4 (10) | 0.103 |
| TV stenosis | 1 (1.5) | 0 (0) | 1 (2.5) | 0.426 |
| TV regurgitation | 21 (32.3) | 7 (28) | 14 (35) | 0.557 |
| MV stenosis | 53 (81.5) | 20 (80) | 33 (82.5) | 0.800 |
| MV regurgitation | 36 (55.4) | 17 (68) | 19 (47.5) | 0.106 |
| ASD | 2 (3.1) | 2 (8) | 0 (0) | 0.069 |
| CPB time, minute | 120 (94 to 145) | 123 (95 to 150) | 118 (92 to 142.5) | 0.526 |
| ACC time, minute | 81 (55 to 100) | 80 (55 to 98) | 83 (60 to 102) | 0.746 |
| Concomitant cardiac surgery | ||||
| MVR | 23 (35.4) | 8 (32) | 15 (37.5) | 0.652 |
| MV repair | 9 (13.8) | 2 (8) | 7 (17.5) | 0.281 |
| OMVC | 4 (6.2) | 1 (4) | 3 (7.5) | 1 |
| MVR and TV repair | 15 (23.1) | 7 (28) | 8 (20) | 0.456 |
| MVR and AVR | 11 (16.9) | 4 (16) | 7 (17.5) | 0.875 |
| MVR and TV repair and AVR | 6 (9.2) | 2 (8) | 4 (10) | 0.786 |
| MVR and AV repair | 3 (4.6) | 1 (4) | 2 (5) | 1 |
| MVR and CABG | 3 (4.6) | 2 (8) | 1 (2.5) | 0554 |
| MVR and LA clot removal | 5 (7.7) | 3 (12) | 2 (5) | 0.365 |
| MVR and PFO closing | 3 (4.6) | 1 (4) | 2 (5) | 1 |
Baseline and Intraoperative Characteristics Based on the Study Groupa
The amount of hospital stay was not significantly different between the groups (13 [9 to 16] in the CMP-IV and 12.5 [9 to 17.5] days in the PVI, P = 0.962). Patients were followed for a median of 16 months (ranging from 3 to 60 months) and no difference was observed between the groups, P = 0.332 (12 [9 to 24] versus 16 [8 to 29] months; Table 2).
| CMP-IV (n = 25) | PVI (n = 40) | P Value | |
|---|---|---|---|
| ICU stay, d | 2 (1 to 2) | 2 (1 to 2.5) | 0.714 |
| Hospital stay, d | 13 (9 to 16) | 12.5 (9 to 17.5) | 0.962 |
| Complications | 0.297 | ||
| Bleeding | 1 (4) | 2 (5) | |
| Malfunction | 1 (4) | 1 (2.5) | |
| Drainage | 3 (12) | 1 (2.5) | |
| Others | 0 | 4 (10) | |
| Follow-up duration, month | 12 (9 to 24) | 16 (8 to 29) | 0.332 |
Clinical Outcomes During Short-Term and Long-Term Follow-Up in the Study Groupsa
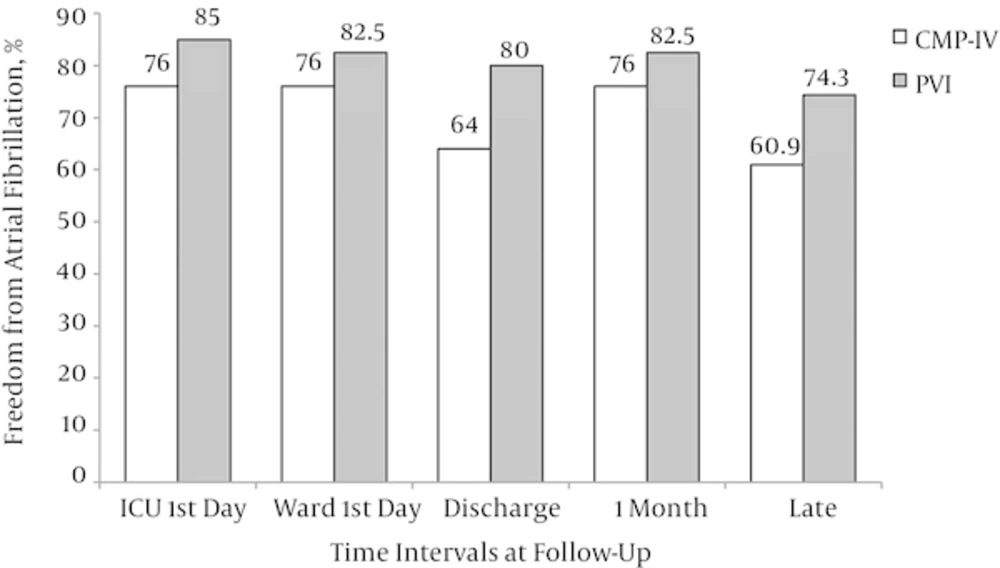
The late follow-up was available in 58 patients (89.2%). The rate of freedom from AF at different intervals, including first day of intensive care unit (ICU) admission, first day of ward admission, at discharge time, 1 month postoperatively, and at late follow-up was not significantly different between the intervention groups (Figure 2). No cases of in-hospital mortality, thromboembolic events, and infective endocarditis were developed. No permanent pace-maker implantation was necessary in our cohort. Mortality and thromboembolic events at late follow-up were not also detected in these patients, although we could not followed up 11% patients at late period.
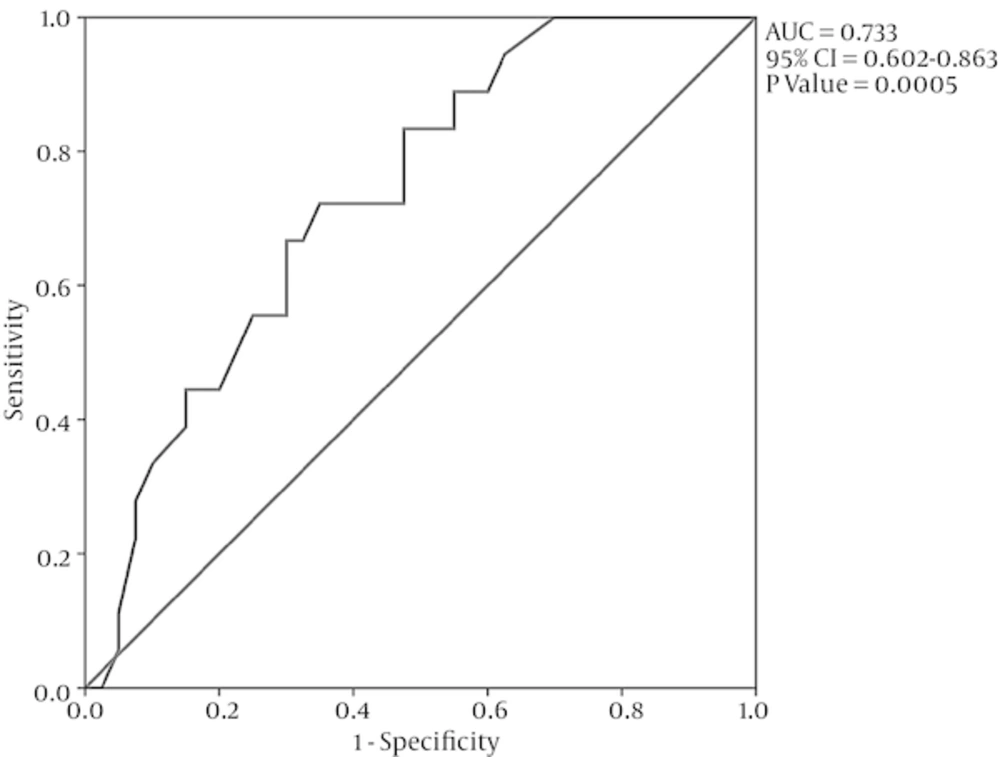
ROC curve analysis was used to detect a cut-off pint for preoperative LA size differentiating the patients with AF development at late follow-up. LA size of 4.5 cm discriminated AF, regardless of the study intervention, with a sensitivity of 89% and a specificity of 45% (area under the ROC curve = 0.733, 95% confidence interval [CI] = 0.602 - 0.863, P = 0.005; Figure 3).
3.2. ROC Curve Analysis
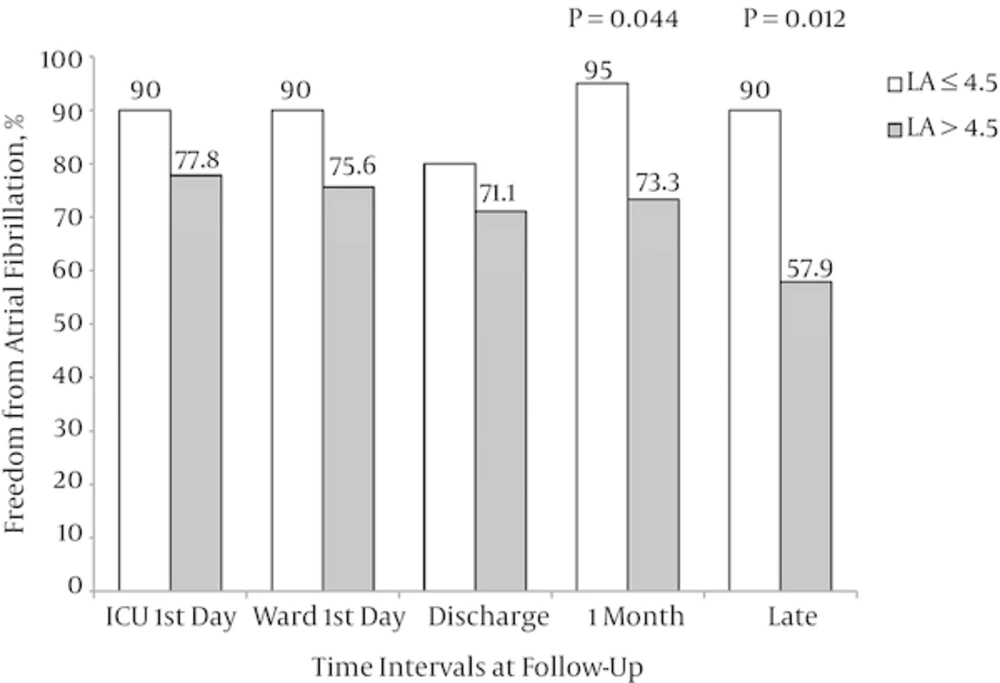
Moreover, based on the cut-off value of 4.5 cm for preoperative LA size, all patients were also divided into two groups (LA ≤ 4.5 [n = 20] and LA > 4.5 cm [n = 45]). There were no significant differences regarding the baseline and intraoperative characteristics between these groups except for AF duration that was significantly greater in the LA > 4.5 group (Table 3). When the rate of freedom from AF during follow-up was compared, the numbers of patients with sinus rhythm at 1-month and late follow-ups were significantly higher in LA > 4.5 group compared with the LA ≤ 4.5 group (Figure 4).
| LA ≤ 4.5 (n = 20) | LA > 4.5 (n = 45) | P Value | |
|---|---|---|---|
| Age, y | 49.5 ± 9.5 | 49.6 ± 11.1 | 0.966 |
| Gender, female | 13 (65) | 31 (68.9) | 0.757 |
| Study groups (CMP IV/PVI) | 5/15 (25/75) | 20/25 (44.4/55.6) | 0.137 |
| AF duration, month | 9 (7 to 17) | 13 (9 to 38) | 0.035 |
| Prior histories | |||
| Diabetes mellitus | 1 (5) | 4 (8.9) | 1 |
| Hypertension | 1 (5) | 7 (15.6) | 0.232 |
| Cerebrovascular events | 2 (10) | 5 (11.1) | 1 |
| Coronary artery disease | 1 (5) | 8 (17.8) | 0.169 |
| Digoxin consumption | 16 (80) | 30 (66.7) | 0.275 |
| Beta blocker use | 10 (50) | 14 (31) | 0.145 |
| Echocardiographic features | |||
| LA size, mm | 3.9 ± 0.4 | 5.6 ± 0.9 | < 0.001 |
| LA clot | 3 (15) | 2 (4.4) | 0.165 |
| LVEF, % | 50 (45 to 55) | 50 (45 to 55) | 0.630 |
| AV insufficiency | 8 (40) | 17 (37.8) | 0.865 |
| AV stenosis | 0 | 4 (8.9) | 0.303 |
| TV stenosis | 0 | 1 (2.2) | 1 |
| TV regurgitation | 5 (25) | 16 (35.6) | 0.401 |
| MV stenosis | 14 (70) | 39 (86.7) | 0.110 |
| MV regurgitation | 10 (50) | 26 (57.8) | 0.560 |
| ASD | 2 (10) | 0 | 0.091 |
| Inotropic use | 2 (10) | 8 (17.8) | 0.422 |
| CPB time, minute | 125.5 (91.5 to 142) | 117 (94 to 147) | 0.086 |
| Aortic cross-clamp time, minute | 91 (55 to 105.5) | 80 (56 to 97) | 0.501 |
| Concomitant cardiac surgery | |||
| MVR | 6 (30) | 17 (37.8) | 0.545 |
| MV repair | 5 (255) | 4 (8.9) | 0.120 |
| OMVC | 2 (10) | 2 (4.4) | 0.581 |
| MVR and TV repair | 1 (5) | 14 (31.1) | 0.021 |
| MVR and AVR | 2 (10) | 9 (20) | 0.321 |
| MVR and TV repair and AVR | 0 | 6 (13.3) | 0.087 |
| MVR and AV repair | 1 (5) | 2 (4.4) | 1 |
| MVR and CABG | 0 | 3 (6.7) | 0.547 |
| MVR and LA clot removal | 3 (15) | 2 (4.4) | 0.165 |
| MVR and PFO closing | 1 (5) | 2 (4.4) | 1 |
| ICU stay, day | 2 (1 to 2.5) | 2 (1 to 2) | 0.155 |
| Hospital stay, day | 14 (9 to 16) | 12 (9 to 16) | 0.214 |
| Complications | 0.348 | ||
| Bleeding | 1 (5) | 2 (4.4) | |
| Malfunction | 1 (5) | 1 (2.2) | |
| Drainage | 1 (5) | 3 (6.7) | |
| Others | 3 (15) | 1 (2.2) | |
| Follow-up duration, month | 20 (12.5 to 29.5) | 12 (8 to 24) | 0.325 |
Baseline Characteristics and Follow-Up Outcomes Based on the LA Size Groupsa
3.3. Multivariate Analysis
In Cox regression analysis, covariates included age, gender, the study intervention groups, preoperative LA size, AF duration, and the number of concomitant surgeries (one, two, and three concomitant surgeries). The predictors of AF at late follow-up were the preoperative LA size (Hazard ratio [HR] 1.962, 95% CI 1.175 - 3.276, P = 0.010) and AF duration (HR 1.028, 95% CI 1.002 - 1.054, P = 0.032) (Table 4).
Cox regression Analysis Identifying the Predictors of AF at Long-Term Follow-Up
4. Discussion
In this study we showed that the rate of freedom from AF at late follow-up was similar for both the cryoablative biatrial CMP-IV and the surgical cryo-PVI in patients with rheumatic MV disease associated with long-standing persistent AF undergoing MVR or MV repair with or without concomitant cardiac surgeries. Moreover, a cut-off point of 4.5 cm for the preoperative LA size discriminated late AF with sensitivity and specificity of 89% and 45%, respectively. The preoperative LA size measured by echocardiography and the duration of AF were also found to be the main predictors of AF at late follow-up.
Among various surgical approaches for treating AF, only the CMP has been proved to be the gold standard for surgical treatment of AF. Regarding the application of AF ablation concomitant with MV surgeries, a recent meta-analysis of 9 randomized studies has found that AF ablation along with MV surgeries compared to alone surgery was associated with an increased freedom from AF and decreases in need for permanent pacemaker implantation and thromboembolism events (13). In contrast, Gillinov et al. (14) have demonstrated that freedom from AF was significantly higher in ablation group compared to alone surgery group, although the need for permanent pacemaker implantation was greater in ablation group. On the other hand, none of the patients in our cohort required permanent pacemaker implantation. We think that underlying arrhythmia such as sick sinus syndrome should be investigated in such groups before anti-arrhythmic surgeries. Moreover, the absence of need for pacemaker implantation in our study may be caused by a relative small sample size and the lack of sick sinus syndrome in our patients.
In our center, the only energy source available was cryothermy that has been previously demonstrated to have a success rate of 65% at 1-year follow-up in both the CMP and PVI (15). Ad et al. (16) reported excellent results with an early success rate of 98% and mortality rate of 3.7% by using cryoablative CMP. In a series of cryoablative PVI with concomitant valvular surgeries, the rates of sinus rhythm were 78% and 69% at postoperative time and long-term follow-up, respectively (17). Furthermore, in a recent large-scale randomized study, the rate of sinus rhythm at follow-up was similar for patients undergoing cryoablative PVI or biatrial CMP (14). Despite studies for approving cryothermy source for AF ablation and showing the similar results in the settings of PVI or CMP, further studies are deemed indicated to further clarify the difference between the cryoablative CMP-IV and surgical cryo-PVI during concomitant valvular surgeries.
All patients in our study had rheumatic mitral valve disease that underwent valvular surgeries along with cryosurgical ablations. Although CMP has been an efficient surgical treatment, two studies have reported that early surgical outcomes for AF associated with MV disease, especially rheumatic MV disease, is less effective (18, 19). It seems that the poor CMP outcomes in the setting of rheumatic MV surgery might be attributed to the RHD inflammation and long duration of AF 18. In addition, it has been postulated that progressively decreasing freedom from AF in such patients can derive from progressive rheumatic changes to the atrial wall or degeneration of atrial tissue resulting from pressure and volume overloading which occurs in valvular heart disease (20). Given these points and our findings, although the rate of freedom from AF decreased at late follow-up, but the success rate in our cohort was acceptable and longer follow-up can be more conclusive in this issue.
The preoperative LA size, AF duration, and patients’ age have been found to be the main predictors of success rate at surgical ablation (11, 12, 21). However, Ad et al. (21) showed that the predictors of sinus rhythm at follow-up period were different in mid- and long-term follow-up. They concluded that we need follow-up longer than 5 years to determine the main predictors of success rate in the surgical ablation of AF. In the present study, in line with previous studies, we showed that the main predictors of AF rhythm at late follow-up were the preoperative LA size and duration of AF, although the cryoablation technique and PVI versus the CMP-IV, did not predict procedure failure. Moreover, we found that the cut-off value of 4.5 cm for LA size discriminated AF during long-term period. We did not equalize the preoperative LA size in the study groups; however, based on subgroup analysis the number of patients with LA > 4.5 cm was comparable between the study groups. According to Sunderland et al. (22) there is no evidence to the contrary that LA diameter less than 4.3 cm is associated with complete CMP success, and the preoperative LA diameter greater than 6 cm has 100% sensitivity to discriminate CMP failure. However, in our cohort, two patients with preoperative LA size of < 4.3 cm had AF at late follow-up period and no patients with LA size of > 6 cm had procedure failure too.
4.1. Study Limitations
This study has its limitations. First, it was small cohort of non-randomized patients. Second, the surgically created ablation scheme did not validated with electroanatomic mapping during the study period and no histologic study performed to identify ablation size or lesion depth.
4.2. Conclusions
N2O-based cryoablative procedures for persistent or long-standing persistent AF are safe, efficacious, and cost-effective at restoring sinus rhythm in the setting of rheumatic MV surgery combined with or without other cardiac surgeries, regardless of the surgical cryo-PVI or cryoablative CMP-IV. However, these cryosurgical ablations were less effective in patients with larger preoperative LA size.