1. Introduction
Duchenne muscular dystrophy is an X-linked disease and usually presents in sons of female carriers (1). The incidence of Duchenne muscular dystrophy is 1 in 3500 male newborns. This disease is characterized by weakness of leg, shoulder and pelvic girdle muscles which is presented in the early childhood (2). Cardiac involvement is common in Duchenne muscular dystrophy which is not related to the degree of skeletal presentation; it may occur dominantly, with or without muscular involvement. Cause of death in Duchenne muscular dystrophy are usually heart failure, heart block or tachyarrhythmia (3). Carriers are often asymptomatic but in rare cases they may have only muscular, muscular and cardiac manifestations (4) or even cardiac manifestations without skeletal muscle weakness (5). The possibility of cardiac presentations in Duchenne muscular dystrophy carriers depends on age (about 15% in female younger than 16 years of age while 45% in female older than 16 years of age) (6). Cardiac involvement of carriers may vary from asymptomatic cases to end stage heart failure ones (7). The main cause of cardiac presentation in carriers is unknown. In some studies, inactivation of one of the two X-chromosome has been recognized as the etiology (8-10).
2. Case Presentation
A 41-year-old female presented at our hospital because of exacerbation of dyspnea and orthopnea, 10 days before her first visit. She was suffering from dyspnea in the last 5 months. Non-exertional chest pain in this period was another symptom of the patient. She had four pregnancies that were leaded to two abortions and two deliveries. The latest delivery referred to 2.5 months ago. Her firstborn was 17 years old male with confirmed Duchenne muscular dystrophy, in his genetic study. The other important family history of the patient was her father’s disease who died at 31 years old because of heart failure with no further investigations. It is noteworthy that her parents were not relative.
She had no history of deafness and or muscle weakness. In her physical examinations, neurologic and muscular signs were not seen. Her electrocardiogram showed sinus rhythm, interventricular conduction defect and T wave inversion in V3 - V6 leads. Morphology of QRS complex in lead V1 was rS. The laboratory data showed that serum Cr: 0.7mg/dL, AST: 20 IU/L, ALT: 4 IU/L, ALKP: 217 IU/L, Bilirubin: 1.1 mg/dL, TSH: 0.9 µIU/mL, pro-BNP: 3415 pg/mL, troponin: 5.1 µg/L (normal range) and creatine kinase (CK): 340 IU/L (normal range).
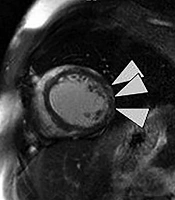
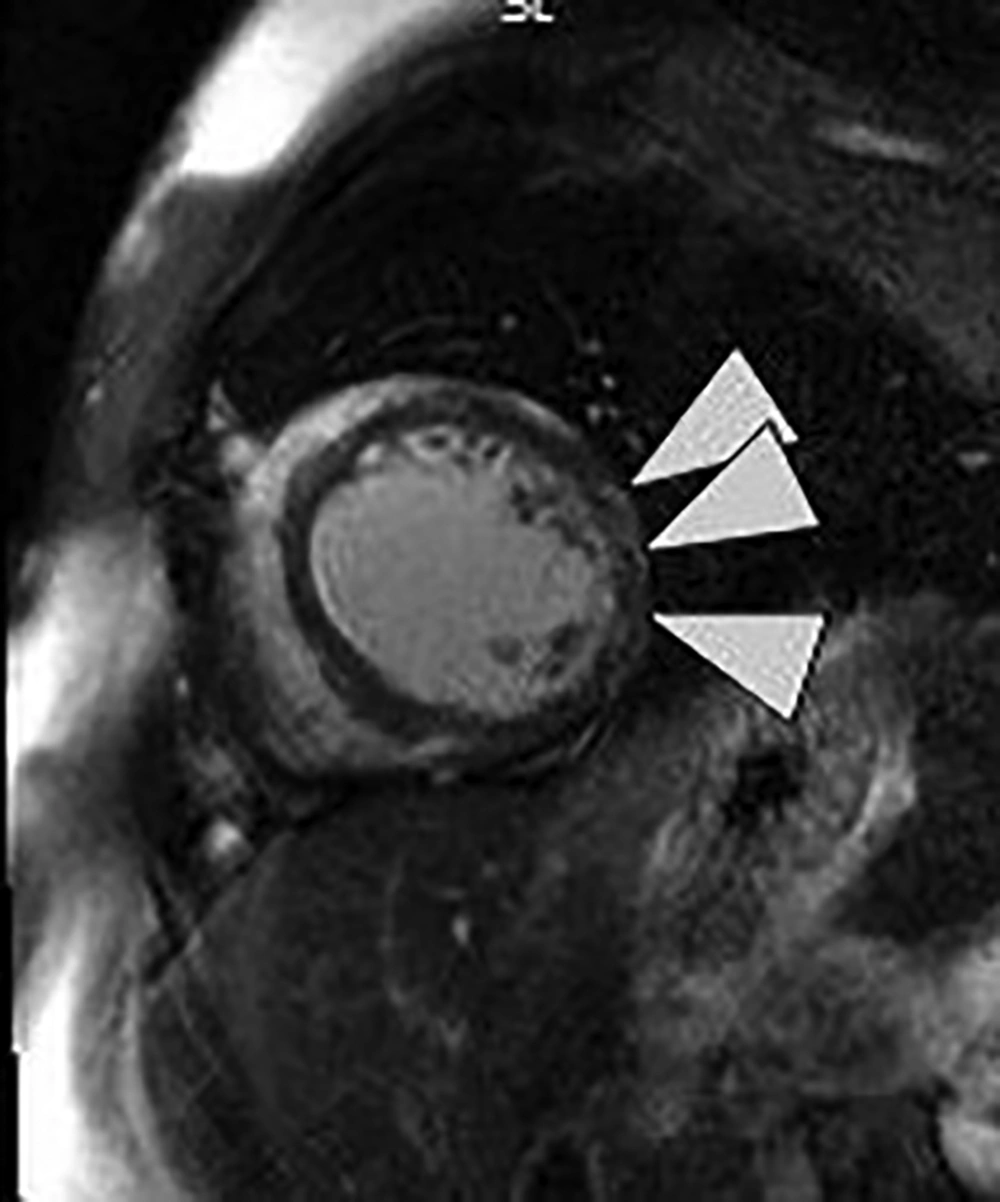
After performing echocardiography, it was revealed that left ventricle (LV) was is moderately enlarged (LVEDVI: 75 cc/m2) with global hypokinesia and severely reduced systolic function (ejection fraction: 10%). Cardiac output was estimated 4600 cc/min. Mitral valve was normal with up to moderate functional regurgitation. In angiography, normal coronary arteries were observed. For further evaluation and specifying the cause of cardiomyopathy, cardiac MRI (CMR) was requested. The CMR results showed severe enlarged LV size with severely reduced systolic function (LVEDVI: 138 mL/m2, LVEF: 13%), normal right ventricle size (RV) with moderate to severe systolic dysfunction (RVEDVI: 59 mL/m2, RVEF: 30%), myocardial hyperemia without edema and there was also evidence of sub-epicardial fibrosis of lateral LV wall (Figure 1). According to CMR findings, muscular dystrophy with prominent cardiac involvement was considered.
The patient was subjected to treatment with Lisinopril (5 mg daily), metoprolol succinate (23.75 mg BID), spironolactone (25 mg daily), furosemide (40 mg TDS), folic acid (1 mg daily) and vitamin B1 (300 mg daily).
3. Discussion
Cardiac and muscle involvement may be seen in female carriers of Duchenne muscular dystrophy. The age of symptom presentation is variable but it almost occurs in adult age (11). Finsterer et al. (12) presented a case of muscular dystrophy carrier, with cardiomyopathy and a rise in serum CK while the increase of CK level is not representative of severity or progression of the disease (13). Florian et al. (14) reported a case of cardiomyopathy without muscular involvement, in a carrier of Duchenne muscular dystrophy while the serum CK level was elevated.
The case presented in this study, had cardiomyopathy without skeletal muscle weakness. Her serum CK level was normal and the disease manifested 2.5 months after delivery. Cheng and Prior also presented a case of asymptomatic Duchenne muscular dystrophy which manifested with post-partum cardiomyopathy (15). CMR, using late gadolinium enhancement is a robust tool for screening of cardiac involvement and early detection of myocardial fibrosis in Duchenne muscular dystrophy patients and carriers. The pattern of fibrosis in their CMR, is presented with subepicardial fibrosis in the inferolateral wall (16). Cardiac MRI findings were in favour of cardiomyopathy due to Duchenne muscular dystrophy. Three differential diagnoses may be considered for this case. First, the patient is a case of familial dilated cardiomyopathy (DCM) which had progression during pregnancy and delivery; second, the patient is a case of postpartum cardiomyopathy and third; the patient is a carrier of Duchenne muscular dystrophy that her disease was progressed during pregnancy and delivery. Pregnancy and delivery may aggravate muscular and cardiac manifestations of carriers (17).
Six months later at follow up, the symptoms including dyspnea and orthopnea were improved and ejection fraction was increased to 25%. To differentiate the diagnosis of this patient, genetic study was recommended but the patient refused it.
Therefore, it is recommended that mothers of sons with Duchenne muscular dystrophy should be screened with CMR and echocardiography at the time of diagnosis of disease in their children (18, 19). They should also follow up using echocardiography every 5 years (20).