1. Background
The prevalence of heart failure is increasing and the rate of admission due to decompensated heart failure (DHF) is high. Many studies were done to evaluate the reasons of frequent admissions in these patients. Inappropriate treatment, limited capacity of hospitals for patients to be admitted, and worsening of the patients’ condition were some of the reasons for readmissions due to DHF morbidity of the patients. In addition the cost of treatment is high. Many guidelines have been written for diagnosis and management of patients with DHF. Registry studies are valuable tools to guide diagnosis and management of heart failure (1).
Stage D or advanced heart failure is the end stage of the disease. According to the American Heart Failure society, advanced or stage D heart failure was defined as persistent and/or progressive severe heart failure signs and symptoms despite optimal medical, surgical, and device therapy. Patients in this phase are symptomatic most of the time (NYHA function class IV) and many of them are resistant to the treatments. Fluid retention, arrhythmias, and heart failure complications including renal failure, electrolyte abnormalities, side effects of the drugs and pulmonary thromboembolism are some of the reasons for readmission (2-4).
The estimated proportion of patients with stage D heart failure is about 5 - 10% of all heart failure population (5) and the studies regarding these patients, their clinical characteristics, prognosis and para-clinical evaluations are limited. Heart transplantation, mechanical circulatory devices and chronic palliative therapies such as intermittent inotropic therapy are the main managing programs in these patients (5-7) so identifying the course of disease for referring the patient for these end stage treatments plays the main role in the management of these patients.
The prediction of disease severity and mortality in heart failure patients could be performed by two widely used models, including, Heart Failure Survival Score (HFSS) and Seattle Heart Failure Model (SHFM). HFSS is mainly based on exercise tolerance of patients besides other factors, regarding inability of stage D patients for performing exercise; this model seems to be inappropriate for these patients. In contrast, SHFM seems to be more practical in these circumstances. One of the important aspects of this mortality prediction by these models is considering patients for advance treatments like Left Ventricular Assisted devices (LVAD) or heart transplant. For example, if one and two-year mortality are more than 38% and 61% respectively, LVAD could be considered for patient (8). However, these models have been validated in a specific population like American people, so generalization of these results may be impractical, and this issue implies necessitation of native studies.
2. Objectives
The present study aimed to characterize Iranian people with advanced heart failure and determine the prognostic predictors of these patients.
3. Methods
3.1. Patient Selection
In this study the patients with advanced heart failure (Stage D) who were admitted in our hospital, were selected from January 2012 to October 2016 among patients registered in Rajaie Acute Systolic Heart Failure (RASHF) data registry (9) according to the following inclusion and exclusion criteria:
3.2. Inclusion Criteria
• Age ≥16 years,
• Highly symptomatic patients with severe left ventricle (LV) systolic dysfunction with LV ejection fraction of 30% or less,
• At least two times of admission during the recent year for acute heart failure (AHF).
3.3. Exclusion Criteria
• Hospitalized patients waiting for a long time for heart transplantation.
• The patients who were discharged in less than a day in any index hospitalization.
• The patients who were admitted for other reasons except AHF.
3.4. Data Acquisition
Rajaie Acute Systolic Heart Failure Registry (9), is a prospective study based on the data of hospitalized acute heart failure patients in this center starting from 2012 in Rajaie Cardiovascular, Medical and Research Center (RHC), a tertiary center for cardiovascular medicine and is ongoing.
The data including clinical and past medical history, para-clinical tests (complete blood counts, liver function tests, thyroid function tests, renal function tests, uric acid and electrolytes), electrocardiogram, and echocardiography are collected from patients’ medical records and gathered in a questionnaire and then are recorded in the software designed by the medical Information Technology (IT) team of RHC. The recorded data are controlled by trained general practitioner and an expert cardiologist every day. This study was approved by the Institutional Research and Ethics Committee of RHC and written informed consents were obtained from all participants.
The endpoint of study was mortality and included patients who were followed for 2 years to report any in-hospital or outpatient mortality till October 2018.
The patients with unknown follow-up in our registry system were followed on the phone.
3.5. Statistical Analysis
For statistical analysis IBM SPSS statistics 19 for Windows (IBM Corp, Armonk, NY, USA) was used. Data are presented as frequencies, mean (standard deviation, SD) or median (interquartile range, IQR) as appropriate. One-sample Kolmogorov-Smirnov test was used to assess the normal distribution of variables. Students’ t-test, paired t test or Mann-Whitney U-test and χ2 test or Kruskal-Wallis tests or Wilcoxon signed rank test were used for comparisons and associations as appropriate. Binary multivariable regression analysis with step-wise selection method was used to define the independent predictors. P value of less than 0.05 was regarded as significant.
4. Results
This study is the first registry to evaluate the patients with advanced heart failure in Iran. Out of the 621 medical records in RASHF registry that were evaluated, only 178 patients met our inclusion criteria, from which we only had access to the follow-up of 143 patients (23 patients were not reachable and 12 patients who underwent transplant entered the transplant registry). Table 1 depicts the patient demographic and clinical characteristics at the time of the enrollment. Most patients were male and the most common etiology for heart failure was ischemic heart disease with or without valvular heart disease.
| Variables | Values |
|---|---|
| Age, y, mean ± SD | 56.9 ± 18.2 |
| Male gender, No. (%) | 132 (74.2) |
| Heart failure duration, years, median (IQR) | 5 (3 - 7) |
| Underlying heart disease, No. (%) | |
| Ischemic cardiomyopathy | 34 (19.1)b |
| Dilated cardiomyopathy | 36 (20.2) |
| Hypertensive cardiomyopathy | 2 (1.1) |
| Valvular cardiomyopathy | 14 (7.9) |
| Chemotherapy induced cardiomyopathy | 2 (1,1) |
| Peri partum cardiomyopathy | 4 (2.2) |
| Muscular dystrophy | 2 (1.1) |
| History of myocarditis | 1 (0.6) |
| Ischemic cardiomyopathy and valvular heart disease | 80 (44.9) |
| Hypertension and valvular heart disease | 3 (1.7) |
| Morbidities, accompanying conditions, No. (%) | |
| Hypertension | 71 (39.9) |
| Diabetes mellitus | 69 (38.8) |
| Coronary artery disease | 105 (59)c |
| Dyslipidemia | 66 (37.1) |
| Smoking | 56 (31.5) |
| Drug abuse | 21 (11,8) |
| Chronic kidney disease | 72 (40.4) |
| Valvular heart diseases | 132 (74.2)d |
| Atrial fibrillation | 44 (24.7)d |
| Transient ischemic attack | 14 (7.9) |
| Peripheral artery disease | 17 (9.6) |
| Alcohol abuse | 2 (1.1) |
| Devices | 67 (37.9) |
Abbreviations: SD, standard deviation; y, years.
a n = 143.
b P value of 0.008 (in relation to mortality).
c P value of 0.005 (in relation to mortality).
d P value of 0.02 (in relation to mortality).
Table 2 shows some clinical, echocardiographic and laboratory characteristics of study population at the time of the diagnosis of heart failure (stage C) compared to findings at the time of the enrollment (Stage D). The progression of the disease is obvious by comparing these variables. For example, LVEF and right ventricular (RV) function have significantly decreased and the patients have developed symptoms and signs of RV failure, hyponatremia and diminished kidney function.
| Characteristics | At Baseline (Stage C) | At Follow-up (Stage D) | P Value |
|---|---|---|---|
| LVEF %, mean ± SD | 19.2 ± 9.4 | 14.4 ± 5.6 | < 0.001 |
| Severe RV dysfunction, No. (%) | 39 (20.8) | 79 (43.3) | < 0.001 |
| Symptoms and signs of RV failure, No. (%) | 43 (23) | 81 (44.4) | < 0.001 |
| Serum sodium level, mg/dL, median (IQR) | 136 (133 - 140) | 136 (132 - 139)b | 0.02 |
| Serum Uric acid level, mg/dL, median (IQR) | 8 (7 - 9) | 8 (6 - 10) | 0.1 |
| Serum BUN, mg/dL, median (IQR) | 19 (16 - 21) | 21 (16 - 31) | 0.006 |
| Serum creatinine, mg/dL, median (IQR) | 0.9 (0.8 - 1.1) | 1 (0.8 - 1.2) | 0.003 |
Abbreviations: BUN; blood urea nitrogen , EF, ejection fraction; RV, right ventricular; SD, standard deviation.
a n = 143.
b P value of 0.07 (in relation to mortality).
Table 3 shows the medications of the study population at enrollment. As shown in table 3 most of the patients received guideline directed medical therapies (GDMT).
| Variables | No. (%) |
|---|---|
| Furosemide | 169 (94.9) |
| Metolazone | 59 (33.1) |
| Beta blockers | 148 (84.6) |
| Angiotensin-converting enzyme inhibitors | 111 (63.4) |
| Angiotensin receptor blockers | 24 (13.7) |
| Mineralocorticoid receptor antagonists | 134 (76.6) |
| Digoxin | 48 (27.4) |
| Calcium channel blockers | 7 (4) |
| Hydralazine | 67 (38.3) |
| Isosorbide dinitrate | 40 (22.9) |
| Aspirin | 69 (39.4) |
| Clopidogrel | 17 (9.7) |
| Warfarin | 66 (37.7) |
| Antiarrhythmic drugs | 22 (12.6) |
| Ivabradine | 6 (3.4) |
| Statins | 47 (26.9) |
| GDMT | 168 (96) |
| Intermittent inotrope therapy | 36 (20.6)b |
| Combined diuretic therapy | 39 (22.3) |
| Intermittent diuretic therapy | 155 (88.6) |
Abbreviation: GDMT, guideline directed medical therapy.
a n = 143.
b P value of 0.04 (in relation to mortality).
Regarding the palliative care of advanced heart failure 89%, 21% and 22% of patients were on intermittent intravenous diuretic therapy, intermittent inotrope therapy and combined diuretic therapy respectively
4.1. The Study Outcome and Its Correlates
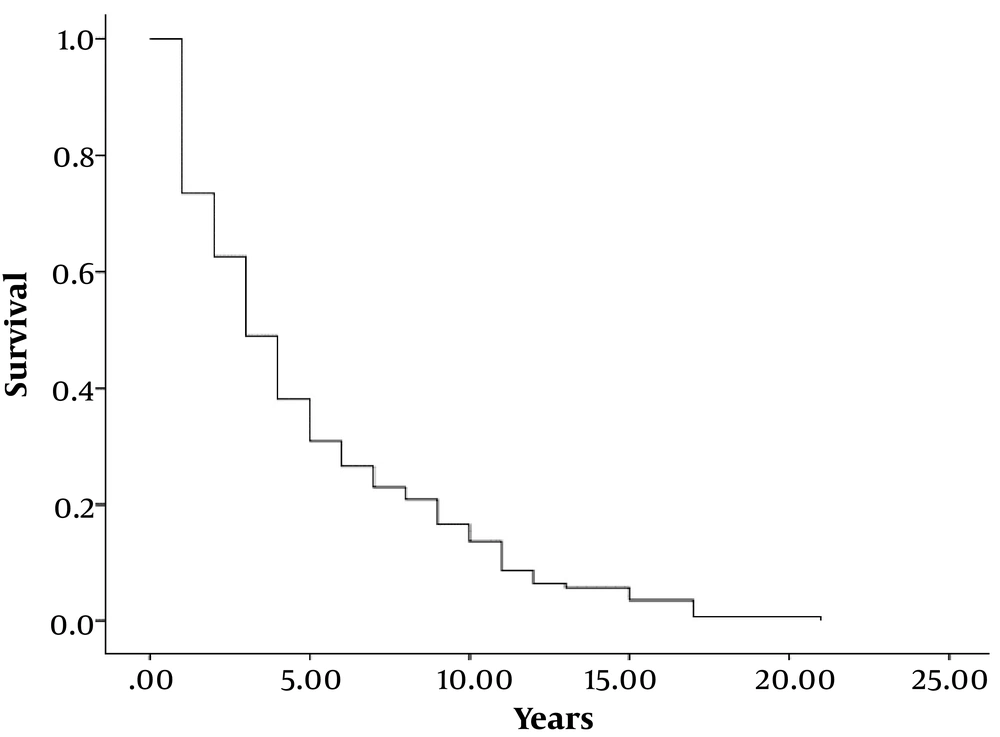
The total mortality was 100 patients (69.9%) at the end of the study. Figure 1 shows the Kaplan Meier curve of survival analysis in our study population. As shown in this curve, about 50% of patients died after 5 years of diagnosis of their heart failure.
Among the study variables, presence of ischemic etiology for heart failure, valvular heart disease, wide QRS, atrial fibrillation, anemia and history of intermittent inotrope therapy were significantly correlated with mortality in univariate analysis (as shown in tables 1-3 all P values were < 0.005).
Binary logistic regression multivariate analysis showed that female gender, anemia and ischemic cardiomyopathy as underlying heart disease have a significant relationship with mortality (Table 4).
| Variables | Beta | P Value | Odd Ratio (95% CI) |
|---|---|---|---|
| History of valvular heart disease | -1.232 | 0.1 | 0.292 (0.07 - 1.3) |
| Ischemic cardiomyopathy | -1.237 | 0.05 | 0.290 (0.08 - 1) |
| Anemia | -2.026 | 0.004 | 0.132 (0.03 - 0.5) |
| History of intermittent inotrope therapy | -1.468 | 0.123 | 0.230 (0.04 - 1.5) |
5. Discussion
The present study showed the clinical characteristics and predictors of death in patients with stage D heart failure in Iranian population. Definition of stage D heart failure is somewhat challenging and investigators have different opinions regarding the best tool for defining these patients. For example, in Hedley et al. study, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile was superior than the European heart failure association criteria in distinguishing stage D heart failure patients in ambulatory heart failure patients with reduced ejection fraction (10). In another study the physician judgment was stronger than existing criteria for defining the prognosis in stage D heart failure (11).
The importance of stage D definition is due to distinct management of these patients in statement of guidelines. According to the American heart failure society, advanced or stage D heart failure was defined as persistent and/or progressive severe heart failure signs and symptoms despite optimal medical, surgical, and device therapy (4, 10, 11).
Transition to stage D or advanced heart failure is the time when the patient is considered for advance heart failure therapies like heart transplant, mechanical circulatory support (MCS) devices or frequent inotrope therapy (3). The MCS are not only important for supportive care in end stage heart failure but also it has been shown that, with optimal guideline directed medical therapy along with the LVAD, some patients could experience remission from stage D (12-14). As MCS devices are not readily available for all patients in our country, we decided to investigate the clinical and para-clinical variables in patients who were on medical and palliative therapies for advanced heart failure such as intermittent intravenous diuretic and inotrope therapy and show the natural history of these patients from the beginning of their heart failure to death. For this purpose, we also excluded the patients who were transplanted or waiting to be admitted for heart transplantation.
It has been shown that heart failure classification from A to D has perceptual, bio-hormonal and prognostic importance (15).
In this study, we could show, besides significant reduction in LVEF, many patients developed signs and symptoms of RV failure and significant deterioration in their renal function and electrolyte balance as indicated in Kalogeropoulos et al. study (3). They showed that variables such as non-ischemic cardiomyopathy, lower initial systolic pressure and LVEF, liver or renal dysfunction, presence of chronic lung disease and blood urea nitrogen may be correlated with early progression to stage D.
Although the proportion of stage D heart failure patients has not been well determined but the estimated rate is 5 to 10% (5).
Patients’ characteristics of this stage have been evaluated in some studies. In Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM), stage D patients were younger, majority of them were male and had a history of coronary artery disease (CAD), chronic kidney disease (CKD) and dyslipidemia (2). In our study, majority of patients were male (74.2%) and had a history of CAD (59%). The prevalence of CKD was high (40.4%) and the prevalence of other known comorbidities such as hypertension (39.9%), diabetes mellitus (38.8%) and dyslipidemia (37.1%), were also prominent.
Some laboratory findings have been shown to have predictive values in stage D heart failure, elevated levels of brain natriuretic peptide (BNP) at admission or follow-up, hyponatremia and elevated blood urea nitrogen (BUN) level are among them (16-19).
In the present study, although there were significant changes in BUN, serum sodium level and ventricular function from baseline to the end of the follow-up, these variables were not correlated with mortality in uni- and multi-variable analyses. The significant predictors of mortality in univariate analyses were the presence of ischemic etiology for heart failure, valvular heart disease, wide QRS, atrial fibrillation, anemia and history of intermittent inotrope therapy, whereas multivariable analysis showed female gender, anemia and ischemic cardiomyopathy may be the independent predictors of death in this group of patients.
The estimated median life expectancy of patients in stage D is about 6 to 12 months, in this phase palliative care for these patients is another issue particularly for patients who are not eligible for mechanical assist devices or heart transplant (6).
Previous mega trials on heart failure patients with New York Heart association (NYHA) function class IV, like Cooperative North Scandinavian Enalapril Survival Study( CONSENSUS), randomized Aldactone evaluation study (RALES), Beta Blocker Evaluation in Survival Trial (BEST), Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS), Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) and Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION), showed high one-year mortality , ranging from 11.4% to 45%, even in the intervention groups (8).
Survival rate of inotrope dependent patients was approximately 10% at 1 year in INTREPID trail (20). In Olmstead county study, stage D heart failure patients had 20% 5-year survival (15).
In our study, 5-year survival rates of patients were less than 50%. These differences in survival rates of stage D patients may be due to various stages in these patients that lead to various survival rates. It might be more practical that stage D patients be divided to more categories based on their clinical, laboratory and other useful findings for precise estimation of their outcomes.
In this regard, some risk prediction models have been designed for survival estimation such as SHFM (Seattle Heart Failure Model), HFSS (Heart Failure Survival Score) which uses peak VO2 in addition to other clinical parameters, ESCAPE risk model and also risk model derived from EFFECT study (21-24).
Prognosis estimation models like SHFM (Seattle Heart Failure Model) are unable to predict exact prognosis of stage D patients, because these patients were not included in most data deriving studies for designing these models and as a result these models underestimate actual prognosis of these patients.
5.1. Study Limitation
Although the uniform nature or study population would be the strength of this study, we could not have the data regarding the pro-BNP level which is one of the most important variables in heart failure studies in our study. The reason was the presence of numerous missing data regarding the pro BNP test results in our documents which was due to unavailability of this test before 2015 in our center.
5.2. Conclusions
Data about stage D heart failure patients are limited. The mortality rate for such patients is relatively high and there’s no clear best treatment approach. Large registries and data acquisition of these patients could be helpful in better management approaches.