1. Background
Metabolic syndrome (MetSyn) includes a set of risk factors for coronary artery disease. According to the National Cholesterol Education Program Adult Treatment Panel III, five indicators are defined for metabolic syndrome, including abnormal values for waist circumference, hypertension, hyperglycemia, elevated triglycerides, and reduced high-density lipoprotein (HDL) cholesterol. According to the above-mentioned definition, the affected person must have at least three of the above factors at the same time. The underlying causes of metabolic syndrome can be overweight and obesity, insulin resistance, unhealthy dietary patterns, physical inactivity, genetic factors, and aging (1).
The metabolic syndrome commonly occurs worldwide, ranging in prevalence from 10% to 40% (2). People with metabolic syndrome are two times more likely to die and three times more likely to have a heart attack or stroke compared to those with no metabolic syndrome (3-5). The overall prevalence rate of metabolic syndrome in Europe was reported to be 15% (6). In a systematic study performed in Asian countries, the prevalence rate of metabolic syndrome was reported to be 19% - 37.2% in men and 13.5% - 42.7% in women, while according to the IDF definition, the prevalence of the syndrome was 18.4% - 36.2% in males and 16.1% - 45.9% in females (7). Moreover, the prevalence rate of metabolic syndrome in Iranian males was reported to be 24%. It appears to be a stronger association between metabolic syndrome and coronary artery disease among middle-aged men than among women, which may be due to the protective role of estrogen (8). This syndrome is also associated with the increased risks of cardiovascular diseases, diabetes, dyslipidemia, stroke, and osteoarthritis. Besides, it gives rise to an increase in some types of cancer, as well as mortality, and imposes high costs on the health care system (9). Therefore, the clinical diagnosis of metabolic syndrome can be considered a valuable tool for high-risk patients, some of whom are patients with hemophilia.
Hemophilia is a rare inherited sex-linked disease caused by a deficiency, or lack of some blood coagulation factors, and its severity will vary depending on the levels of these factors in the blood (10). Cardiovascular disease in patients with hemophilia is regarded as an emergency condition (11). The complexity of establishing a delicate balance and determining the risk of bleeding against the risk of atherosclerosis in these patients raises the question of their treatment (12). Patients with hemophilia appear to be protected against cardiovascular disease due to their hypercoagulation status (13). However, according to some statistics, cardiovascular disease is one of the most common causes of death among patients with hemophilia (14). Moreover, in some studies, the risk of these diseases has been reported to be the same in people with hemophilia and normal people (11).
Nowadays, advances in the care of these patients have increased their life expectancy from less than 30 to more than 70. With increased life expectancy, cardiovascular disease has been reported to occur to a greater extent. On the other hand, it is stated that, in these individuals, the known risk factors for cardiovascular disease are independently associated with the prevalence of this disease (11). Therefore, the identification of high-risk individuals among patients with hemophilia is essential and can be effective for controlling and reducing cardiovascular disease in these patients. Accordingly, there is little information available on the prevalence of metabolic syndrome in patients with hemophilia compared to normal people.
Therefore, this study was designed to determine the prevalence of metabolic syndrome among patients with hemophilia who need awareness of the prevalence of this syndrome and identify high-risk individuals to initiate preventive measures.
2. Methods
A case-control study was performed on 91 patients with records in the Hemophilia Society of Southern Khorasan in 2015. The hemodialysis group was selected based on a census from all people with records in the hemophilia center. Also, 91 healthy individuals who did not have hemophilia were randomly selected from healthy individuals in Birjand City (15). The two groups were matched for age and sex and then underwent the same examinations.
The study protocol was approved by the Human Research Committee of the Birjand University of Medical Science (no.: ir.bums.REC). All patients completed the consent forms before participating in the study after receiving explanations about the forms of ethical consent. All the hemophilia patients with no history of heart disease participated in the study. They were selected regardless of the severity of the disease.
A questionnaire and a clinical survey were used to obtain the information needed for this study. Data were then collected using a researcher-made checklist consisting of demographic information and some risk factors for heart disease. After obtaining informed consent from individuals, the researcher explained the aims of the research to the individuals and assured them that the obtained information would remain confidential. Data were collected by a trained student and nurse. In addition, blood pressure, weight, height, and waist circumference were measured using standard methods. Patients’ blood pressure was measured using a mercury barometer, with the cuff proportional to their arm circumference such that the barometer cuff covered two-thirds of the arm surface in a sitting position for the right arm. A digital scale was used, which was checked for each weight measurement and then calibrated at regular intervals. Weight was measured with minimum clothing and no shoes using a digital scale with 100 grams accuracy. The BMI was calculated using the following formula: weight in kilograms divided by height in meters squared. Moreover, HDL levels were considered to be low when they were below the fifth percentile of the reference values of the concerning age categories. Venous blood (8 mL) was also collected from each subject. Afterward, the sera were placed in sterile-covered storage tubes and stored at -30°C until the collection of all samples. The results were reported to the subjects. After 12 hours of fasting, the sera were sent to the Laboratory of Valiasr Hospital to measure serum glucose and lipids. The measurements were performed by standard Lab kits.
Data analysis was performed using SPSS19 software. Descriptive indicators, including frequency, mean, standard deviation, and median, were reported. Moreover, normality was assessed using the Kolmogorov-Smirnov test. Afterward, to compare the mean values in the two groups, the independent sample t-test and Mann-Whitney test were used. For qualitative variables, the chi-square and Fisher tests were used. A level of 5% was considered statistically significant.
3. Results
This case-control study enrolled 91 patients with hemophilia, as well as 91 healthy subjects in the control group. The mean age was 34.11 ± 14.68 and 33.72 ± 13.46 years in hemophilia and control groups, respectively. The results of the independent sample t-test showed no significant difference in the mean age between the two groups (P = 0.85).
In the hemophilia group, 84 (92.3%) subjects were male, and 7 (7.7%) subjects were female, while in the control group, 84 (92.3%) subjects were male and 7 (7.7%) subjects were female. The results of the chi-square test showed that gender did not significantly differ between the two groups (P = 1.00).
In hemophilia and control groups, 49 (53.8%) subjects were aged between 20 and 39 years, 25 (27.5%) subjects were aged ≥ 40 years, and 17 (18.7%) subjects were aged < 20 years. The results of the chi-square test showed that the age did not significantly differ between the two groups (P = 1.00).
There was no statistically significant difference between the two groups of hemophilia patients and healthy subjects in terms of gender, marital status, age, and occupation (P > 0.05) (Table 1). The Fisher exact test results showed a statistically significant difference between the two groups in the level of education (P = 0.004), and the control group subjects were more literate. The results of the Fisher exact test also showed a statistically significant difference between the two groups in smoking, and the rate of smoking was significantly higher in the hemophilia group than in the healthy group (P = 0.04) (Table 1).
| Variable | All | Hemophilia Group | Control Group | Result |
|---|---|---|---|---|
| Marital status | χ2 = 1.088; P = 0.747 | |||
| Unmarried | 54 (29.7) | 26 (28.6) | 28 (30.8) | |
| Married | 127 (69.8) | 65 (71.4) | 62 (68.1) | |
| Widowed | 1 (0.5) | 0 (0) | 1 (1.1) | |
| Job | χ2 = 7.433; P = 0.115 | |||
| Employee | 23 (12.6) | 8 (8.8) | 15 (16.5) | |
| Housewife | 11 (6) | 5 (5.5) | 6 (6.6) | |
| Self-employed | 92 (50.5) | 49 (53.8) | 43 (47.3) | |
| Student | 31 (17) | 12 (13.2) | 19 (20.9) | |
| Unemployed or retired | 25 (13.7) | 17 (18.7) | 8 (8.8) | |
| Level of Education | Fexact = 15.345; P = 0.004b | |||
| Illiterate and literate | 13 (7.2) | 9 (9.9) | 4 (4.4) | |
| Elementary | 39 (21.4) | 26 (28.6) | 13 (14.3) | |
| Secondary | 40 (22.0) | 20 (22 ) | 20 (22) | |
| High school | 54 (29.7) | 27 (29.7) | 27 (29.7) | |
| Academic | 36 (19.8) | 9 (9.9) | 27 (29.7) | |
| Smoking | Fexact = 3.59; P = 0.04b | |||
| Yes | 20 (11) | 14 (15.4) | 6 (6.6) | |
| No | 162 (89) | 77 (84.6) | 85 (93.4) |
aValues are expressed as No. (%).
bSignificant.
The median (interquartile range) LDL levels were 92 (72 - 119) and 127 (100 - 152) in hemophilia and control groups, respectively. The results of the Mann-Whitney U test showed that the LDL levels were significantly higher in healthy subjects than in hemophilia patients (P < 0.001). The results also showed that mean TG (P = 0.016) and waist circumference (P = 0.001) were significantly higher in the healthy group than in the hemophilia group. The mean cholesterol level was significantly higher in the healthy group than in the hemophilia group (185.27 ± 39.9 vs. 159.65 ± 35.88, P < 0.001) (Table 2).
| Variable | Hemophilia Group | Control Group | Result |
|---|---|---|---|
| Chol | 159.65 ± 35.88 | 185.27 ± 36.90 | t = 4.57.14, P < 0.001b |
| FBS | 91 (86 - 98) | 90 (84 - 98) | Z = 0.224, P = 0.823c |
| TG | 110 (88 - 135) | 120 (95 - 187) | Z = 2.41, P = 0.016 |
| HDL | 37 (33 - 43) | 37 (32 - 42) | Z = 0.075, P = 0.94 |
| LDL | 92 (72 - 119) | 127 (100 - 152) | Z = 5.047, P < 0.001 |
| Waist | 83.09 ± 13.45 | 89.02 ± 10.01 | t = 3.368, P = 0.001 |
| SBP | 120 (110 - 125) | 120 (115 - 120) | Z = 1.401, P = 0.161 |
| DBP | 80 (70 - 80) | 80 (70 - 80) | Z = 0.992, P = 0.321 |
| BMI | 22.99 ± 4.87 | 24.48 ± 4.14 | t = 2.219, P = 0.028 |
aValues are expressed as mean ± SD or median (Q1-Q3).
bIndependent sample t-test.
cMann-Whitney U test.
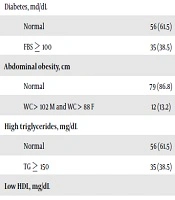
In the hemophilia group, there were 15 individuals with diabetes (16.5%) and eight individuals with abdominal obesity (8.8%), and 16.5% of them showed the triglyceride levels above 150 mg/dl while 70.3% had the HDL levels lower than mg/dL. The chi-square test results showed that the frequency distribution of diabetes and triglyceride was significantly different between the two groups, and they were more prevalent in the healthy group (P < 0.01). Also, metabolic syndrome was 1.12% among healthy subjects and 4.4% among hemophilia subjects (Table 3).
| Variable | Variables | χ2 | P | |
|---|---|---|---|---|
| Control Group | Hemophilia Group | |||
| Diabetes, md/dL | 5.611 | 0.03b | ||
| Normal | 56 (61.5) | 76 (83.5) | ||
| FBS ≥ 100 | 35 (38.5) | 15 (16.5) | ||
| Abdominal obesity, cm | 0.899 | 0.478 | ||
| Normal | 79 (86.8) | 83 (91.2) | ||
| WC > 102 M and WC > 88 F | 12 (13.2) | 8 (8.8) | ||
| High triglycerides, mg/dL | 11.03 | 0.001b | ||
| Normal | 56 (61.5) | 76 (83.5) | ||
| TG ≥ 150 | 35 (38.5) | 15 (16.5) | ||
| Low HDL, mg/dL | 0.027 | 1.000 | ||
| Normal | 26 (28.6) | 27 (29.7) | ||
| HDL< 40 M and HDL < 50 F | 65 (71.4) | 64 (70.3) | ||
| High blood pressure, mmHg | 0.117 | 1.000 | ||
| Normal | 87 (95.6) | 86 (94.5) | ||
| BP ≥ 130/85 | 4 (4.4) | 5 (5.5) | ||
| Metabolic Syndrome | 3.560 | 0.05 | ||
| Yes | 11 (12.1) | 4 (4.4) | ||
| No | 80 (87.9) | 87 (95.6) | ||
aValues are expressed as No. (%).
bSignificant.
4. Discussion
This study aimed to investigate the prevalence of metabolic syndrome and the risk factors for cardiovascular disease in patients with hemophilia referring to the Hemophilia Society of Southern Khorasan in 2015, compared with healthy subjects. According to the results of this study, the prevalence rate of metabolic syndrome was 12.1% among healthy subjects and 4.4% in hemophilia subjects. The results of this study showed a significant difference between the hemophilia group and the healthy group in terms of metabolic syndrome. Also, some components of the metabolic syndrome were different between the two groups, such as diabetes and high triglycerides, which were higher in the healthy group. In a study by Kulkarni et al. (14) conducted on male hemophilia patients and healthy subjects, the prevalence of diabetes was higher among healthy subjects than among hemophilia subjects, which is consistent with the results of our study. However, in the Walsh et al.’s (16) study performed on patients with hemophilia and healthy subjects, the prevalence of diabetes was higher among hemophilia patients than among healthy subjects, which is inconsistent with the results of our study. This may be due to the difference in the mean BMI in hemophilia subjects participating in Walsh et al.’s (16) study, which, in turn, led to a higher prevalence of diabetes.
In the studies performed by Nair et al. (17) and Tlacuilo‐Parra (18), a lower rate of overweight was reported in subjects with hemophilia than among the healthy population. Accordingly, the results of this study are similar to the results of the present study. In our study, the mean BMI was significantly lower in hemophilia patients than in the healthy group. The differences observed in different studies can be attributed to some factors such as genetic differences, nutrition, activity level, and access to medical care.
In a study by Foley et al. (13) and Rosendaal et al. (19), blood pressure was significantly higher in hemophilia patients than in the healthy group. However, in our study, the frequency of hypertension was not significantly different between the two groups. Also, the mean systolic and diastolic blood pressures were not significantly different between the two groups, for which we have no justification.
Another result of the present study was the lower levels of total cholesterol in subjects with hemophilia than in healthy subjects. In line with our results, Rosendaal et al. (19) evaluated the frequency of risk factors for cardiovascular disease in 95 hemophilia patients and reported lower total cholesterol levels in patients with hemophilia. Biere-Rafi et al.’s (20) study also found lower mean cholesterol levels in hemophilia subjects than in the control group and also reported that the severity of hemophilia had no effect on total cholesterol, which is consistent with the results of our study. Also, in the Tuinenburg et al.’s (21) study, which compared the prevalence of cardiovascular risk factors between 100 hemophilia patients and 100 healthy subjects, the mean total cholesterol and high cholesterol ratio in the hemophilia patients were lower than those in the healthy group. Another study reported the lower mean total cholesterol levels in hemophilia subjects (13), which is consistent with the results of our study. There is a hypothesis for the prevalence of low cholesterol in hemophilia patients stating that during their lifespan, patients with hemophilia are severely exposed to foreign proteins that affect their immune system and their liver function is also affected by viral infections. These changes were made due to the treatment with human plasma drugs, which may explain low blood cholesterol levels in these patients (19, 22, 23).
The results of our study showed that the LDL level was significantly lower in subjects with hemophilia and the HDL level was not significantly different between the two groups. In this regard, Sait et al.’s study (24) was performed to investigate the cardiovascular risk factors in patients with hemophilia and showed that the LDL level in hemophilia patients was lower than that in healthy subjects. Also, they reported a statistically significant difference between the two groups studied in terms of HDL levels, the results of which are consistent with the results of our study (24).
In our study, the prevalence of metabolic syndrome was lower in hemophilia subjects than in the control group, which may be justified given that its constituents include abdominal obesity, hypertension, high blood sugar, high triglyceride, and low HDL. As noted above, these factors were lower in most of the hemophilia patients in many studies (14, 17-19, 21).
In this study, the cardiovascular risk factors that showed the lower incidence of some factors in hemophilia patients were also examined. Other studies have also reported a lower incidence of cardiovascular disease among these patients, such as the Plug et al.’s (25) cohort study of 967 patients with hemophilia, who reported a very low frequency of cardiovascular disease among hemophilia patients.
The low incidence of cardiovascular disease in patients with hemophilia is most likely attributed to changes in the coagulation system of these patients and the formation of less vascular thrombosis (20). They also receive some special care due to chronic illness (monitoring of weight, hypertension, fasting blood sugar, and lipid profile), leading to better control of lifestyle.
4.1. Conclusions
This study showed that the prevalence of metabolic syndrome was significantly lower in subjects with hemophilia than in the control population. Some components of the metabolic syndrome including diabetes and high triglyceride were also higher in the control population than in hemophilia patients; however, no statistical difference was observed between the two groups in terms of blood hypertension. Therefore, the risk of cardiovascular disease was not estimated to be higher in these patients than in the general population.