1. Background
The term complex regional pain syndrome (CRPS) is used to define a variety of painful situations that appear after certain lesions, which manifest regionally and whose location is predominantly distal to one limb. The symptoms exceed in magnitude and in time to the expected course of the causative lesion and produce a significant alteration of the motor function. The pain has a series of characteristics: spontaneity, allodynia, hyperalgesia, edema, autonomic abnormalities and signs of atrophy (1). The incidence of CRPS is high and very variable. Perhaps the most evaluated trigger cause, as it is the most frequent, is the fracture of the distal extremity of the radius. In the Bickerstaff and Kanis study (2), they established an incidence of 28%, which decreases from the first year to 1% - 2%. The incidence is higher in women than men, with a ratio 4:1 (3).
The pathophysiology of CRPS is not known and different hypotheses are raised that attempt to explain a series of phenomena existing in its genesis. It is postulated that its origin could be in the presence of repetitive or sustained harmful stimuli over time, which would be responsible for peripheral and central sensitization phenomena, leading to the appearance of hyperalgesia and allodynia (4). Abnormally, there is a hyperactivity of the sympathetic nervous system, which is manifested by changes in the temperature, coloration, and sweating of the skin in the affected area and by the fact that pharmacological or surgical sympathectomy can relieve pain (5). Trigger factors for CRPS include distal fractures, surgery, sprains, direct trauma to objects, gunshot wounds and animal bites (6). Frequently, there has been immobilization after trauma (6). In 50% of cases they are located in the upper part of the body (arms, neck or shoulders) and in 47% in the legs, hip and lower back (6).
Pain is the main symptom and has characteristics that allow it to be defined as neuropathic pain. Therefore, the diagnosis is clinical, through the fulfillment of the standards of the International Association for the Study of Pain (IASP, 2003). There is no specific diagnostic test (6). On radiography, the most important radiological findings, although non-specific, are the increase in soft tissue and regional osteoporosis. On Gammagraphy, an accumulation of the radionuclide technetium 99% in periarticular regions, greater than the contralateral area is observed, apparently due to an increase in blood flow (7). Magnetic resonance imaging (MRI) with gadolinium enhancement seems to determine that in the acute phase, hyperintense signals appear in T2 and T1, which correspond to the presence of hemodynamic abnormalities caused by microangiopathy and/or abnormalities of the sympathetic nerve system, which can lead to muscular ischemia (8). In an overall view, radiologic and gammagraphic findings are present in both extremities but are more prominent in one of them (7, 8). On neurophysiology, sympathetic skin-response (SSR) is a method to evaluate the involvement and dysfunction of the sympathetic nervous system (9, 10).
The management of CRPS includes pain management, physical therapy (electromagnetic fields, manual therapy) and psychotherapy. For the management of pain, NSAIDs (non-steroidal anti-inflammatory drugs), opioids, antidepressants, and anesthetics are used (11).
Recently, it has been reported that nutraceuticals could inhibit inflammation and stimulate the recovery of nerve function, as in post decompression surgery. In particular, alpha-lipoic acid (ALA) is believed to be of particular interest because of its antioxidant properties caused by an improvement in the activity of glutathione peroxidase. Furthermore, ALA has also demonstrated neuroprotective and neurotrophic properties in many neurologic diseases (12). Another dietary supplement is vitamin C, a recognized anti-oxidant that inhibits reactive oxygen species (ROS) which damage microcirculation and membrane lipids. On the other hand, vitamin C minimizes edema by decreasing exaggerated vascular permeability. Since inflammation and micro-angiopathy are mechanisms involved in CRSP, several studies have postulated the benefits of vitamin C supplementation in the prevention of CRPS (13).
Since the complications of CRPS are associated with dysfunction of the sympathetic nervous system, where the primary inflammatory changes are associated with the generation of oxygen free radicals secondary to disturbances in sympathetic microcirculation; it is expected that ALA and vitamin C could play a role in the management and/or prevention of CRPS, because both exhibit free radical scavenger properties (14).
2. Objectives
The objective of the present study is to demonstrate the efficacy of physical therapy and TIOBEC® (a combination of α-lipoic acid and vitamin B, C, and E) in the management of CRPS in a small series of patients, in terms of pain amelioration and edema improvement.
3. Methods
3.1. Trial Design
This was a prospective before-and-after study that evaluated the effectiveness of physical therapy (electromagnetic fields and manual therapy) plus TIOBEC® on the management of CRPS. Prior to starting patient enrollment, the Ethical Committee of Hospital Universitario Santa Cristina approved the study (code: CE-12/2017). The study was ran from January 2018 to September 2019 and performed at the Rehabilitation Department, Hospital Universitario Santa Cristina, Madrid-Spain. The study agrees with Helsinki's ethical principles and all participants signed Informed consent prior to enter the study. Outcome measures were evaluated at baseline and at mid-term (4 months follow-up period).
3.2. Participants
All consecutive patients from the Rehabilitation and Physical Medicine Department were screened for inclusion.
Inclusion criteria: (1) patients older than 18 years; (2) confirmed diagnosis of CRPS by clinical, radiological and neurophysiological criteria; (3) completed outcome measures (pain scale, edema and functional state). Exclusion criteria: (1) allergy to ALA/vitamin C; (2) chronic pain due to comorbidities; (3) absence of outcome measures.
3.3. Treatment Protocol
The management of CRPS included both physical therapy and oral medication. For physical therapy, electromagnetic fields were applied to the symptomatic limb at a dose of 20 Hz-50 Gauss-20 min (15), followed by manual therapy, in order to recover movement (mobilization) and to decrease edema (manual drainage). Manual therapy included assisted, active and resisted mobility of the hand-fingers/foot-toes plus manual drainage (20 - 30 minutes/day) and it was performed by a physiotherapist. For oral medication, TIOBEC® 400 mg was prescribed two times a day for 3 months. The TIOBEC® components included α-lipoic acid (800 mg), vitamin C (60 mg), niacin (36 mg), vitamin E (10 mg), vitamin B1 (25 mg), vitamin B2 (25 mg),vitamin B6 (9.5 mg), vitamin B12 (25 µg) and folic acid (400 µg) (16).
3.4. Outcome Measures
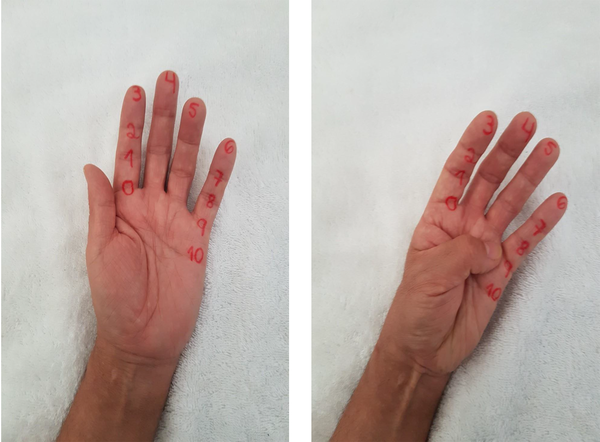
Outcome measures included VAS (visual analog scale) pain scale, reduction of edema and functional Kapandji score, which were evaluated before-and-after treatment. VAS is the preferred instrument to evaluate pain in clinical studies because of its sensitivity, reproducibility, and simplicity. It is a ruler graded from 0 - 10, where 0 means “without pain” and 10 mean “unbearable pain” (17). Edema was evaluated measuring the circumference at the hand/foot, at the level of metacarpal/metatarsal-phalangeal joint, in millimeters (mm) and it was compared to the contralateral hand/foot, considering the difference between the affected and not affected limb. For function evaluation, the Kapandji score was used (18). The Kapandji finger opposition test (Kpandji score) was used to determine motor ability in the CRPS-affected hand. This test involves the subject attempting to touch the thumb on their CRPS-affected hand to 10 points on the same hand in order from points 0 to 10 (second metacarpal-phalangeal joint to fifth metacarpal-phalangeal joint). A 0 Score denotes no opposition, whereas a 10 Score denotes complete opposition (Figure 1).
The Functional Kapandji score denotes opposition of the thumb with the other fingers. A 0 score (thumb to the second metacarpal-phalangeal joint) denotes no opposition, whereas a 10 score (thumb to fifth metacarpal-phalangeal joint) denotes complete opposition (Leamy et al. (18)).
3.5. Statistical Analysis
SPSS 20.0® was used for statistical analysis. In the descriptive analysis at baseline, the mean and standard deviation in the case of quantitative variables, while frequencies and percentages in the case of quantitative variables, were analyzed. Wilcoxon test was performed to assess normality between patients. Therefore, considering a normal distribution and homogeneity of the variances, for the comparison of the treatment group in similar time periods, the non-parametric Mann-Whitney U test was applied with a level of significance established at 0.05 (P < 0.05).
4. Results
A total of 5 patients with CRPS were recruited from January 2018 to September 2019. The mean age of patients was 61 ± 7.9 years. Female patients were more frequent than male patients with a ratio of female to male of 3:2 (Table 1). All of the patients presented previous surgeries and secondary immobilization as the triggering cause of CRPS. All of the patients were diagnosed clinically, and 100% were confirmed by gammagraphy and radiography. All patients were evaluated by neurophysiological sympathetic skin-response (SSR) (Table 1). Mean electrotherapy and manual therapy (electromagnetic fields plus mobilizations and manual drainage) sessions were 40 ± 27.83. Mean Oral medication (TIOBEC®) was 84 ± 13.41 days (Table 1).
| Variables | Values |
|---|---|
| Age, y | 62 ± 7.91 |
| Female gender | 3 (66) |
| Ratio, female:male | 3:2 |
| Drop-outs during study secondary to adverse events | 0 |
| CRPS on upper limb | 4 (80) |
| Clinical diagnosis | 5 (100) |
| Gammagrafic diagnosis | 5 (100) |
| Radiolographic diagnosis | 5 (100) |
| Neurophysiologic diagnosis (SSR) | 5 (100) |
| Initial VAS, 0 - 10 | 6.6 ± 0.54 |
| Initial edema | 12 ± 5.7 |
| Initial functional Kapandji score, 0 - 10 | 6.2 ±2.77 |
| Rehabilitation sessions (physical and manual therapy) | 40 ± 27.83 |
| TIOBEC® treatment | 84 ± 13.41 |
Main Variables of the Study at Baseline (Before Physical Therapy and TIOBEC®)a
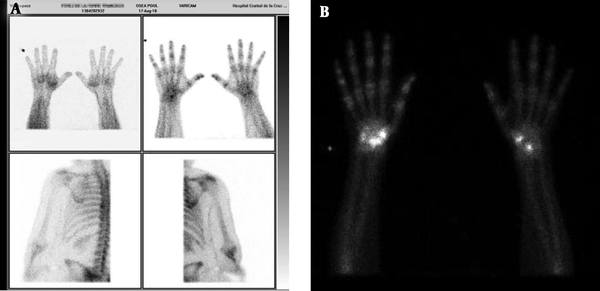
Complete information of 5 cases was as follows. Case 1 was a 69 year-old male who after synovectomy because of De quervain’s tendonitis developed pain, edema (10 mm at the metacarpal-phalangeal joint), erythema and sweating. Symptoms, gammagraphy, and SSR (sympathetic-skin response) were compatible with CRPS (Figure 2A). After 15 sessions of physical therapy and 90 days of TIOBEC® treatment, pain ameliorated. Case 2 was a 71 year-old female who after neurolysis of right median nerve because of carpal tunnel syndrome developed pain, edema (5 mm at the metacarpal-phalangeal joint), erythema and sweating. Symptoms, gammagraphy and SSR were compatible with CRPS (Figure 2B). After 35 sessions of physical therapy and 90 days of TIOBEC® treatment, pain and edema subsided. Case 3 was a 60 year-old female who after osteotomy because of corrective surgery for hallux valgus and metatarsalgia developed pain, edema (20 mm at the metatarsal-phalangeal joint), erythema, sweating and adhered surgical scar. Symptoms, gammagraphy and SSR were compatible with CRPS (Figure 3A). After 85 sessions of physical therapy and 90 days of TIOBEC® treatment, pain and edema decreased. Case 4 was a 52 years-old female who after neurolysis of right median nerve because of carpal tunnel syndrome developed pain, allodynia, edema (10 mm at the metacarpal-phalangeal joint), rigidity (Kapandji score 5/10), erythema and sweating. Symptoms, gammagraphy and SSR were compatible with CRPS (Figure 3B). After 20 sessions of physical therapy and 90 days of TIOBEC® treatment, pain, edema, and rigidity (Kapandji score 10/10) ameliorated. Case 5 was a 58 year-old male who after mini tight rope arthroscopic technique of first metacarpal bone because of left rhizarthrosis developed pain, edema (15 mm at the metacarpal-phalangeal joint), rigidity (Kapandji score 3/10), erythema and sweating. Symptoms, gammagraphy and SSR were compatible with CRPS (Figure 4). After 45 sessions of Physical therapy and 60 days of TIOBEC® treatment, pain, edema, and rigidity (Kapandji score 7/10) subsided.
A, Case 1: 69 year-old male with CRPS after synovectomy because of De quervain tendonitis. Gammagraphy shows the enhancement of radionucleotide Tec99 on the wrist; B, case 2: 71 years-old female with CSRP after neurolysis of the median nerve because of carpal tunnel syndrome. Gammagraphy shows the enhancement of radionucleotide Tec99 on the right wrist.
A, Case 3: 60 year-old female with CRPS after osteotomy because of corrective surgery for hallux valgus and metatarsalgia. Gammagraphy shows the enhancement of radionucleotide Tec99 on the right metatarsal bones; B, case 4: 52 year-old female with CRPS after neurolysis of the median nerve because of carpal tunnel syndrome. Sympathetic-skin response (SSR) shows the absence of inhibition (100% persistence) that denotes hyperexcitability of sympathetic autonomic system.
A, Case 5: 58 year-old male with CRPS after mini tight rope arthroscopic technique of first metacarpal bone because of rizarthrosis. Gammagraphy shows enhancement of radionucleotide Tec99 on some carpal bones at the wrist, first metacarpal and first and second phalanx bones. B, sympathetic-skin response (SSR) shows the absence of inhibition (100% persistence) that denotes the hyperexcitability of the sympathetic autonomic system.
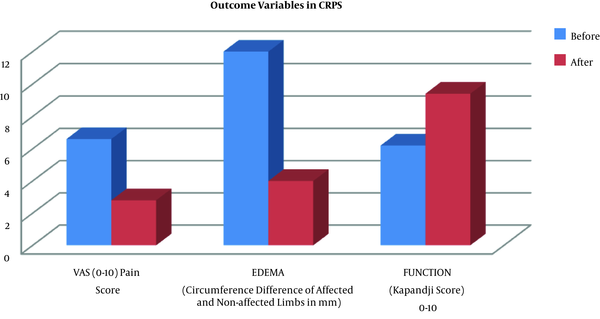
Physical therapy and TIOBEC® decreased pain in CRPS patients from 6.6 ± 0.54 to 2.8 ± 1.3 points (P = 0.0015), edema from 12 ± 5.7 mm to 4 ± 4.18 mm (P = 0.0349) and improved functional Kapandji score from 6.2 ± 2.77 to 9.4 ± 1.34 (P = 0.0299) (Table 2).
| Outcome Variables | Pre-Treatment | Post-Treatment | P |
|---|---|---|---|
| VAS (0 - 10) | 6.6 ± 0.54 | 2.8 ± 1.3 | 0.001 |
| Edema (circometry of affected limb in mm) | 12 ± 5.7 | 4 ± 4.18 | 0.035 |
| Functional Kapandji score ( 0 - 10) | 6.2 ± 2.77 | 9.4 ± 1.34 | 0.029 |
Neither of the patients stopped oral medication nor reported adverse events. Physical therapy and TIOBEC® improved outcome measures in an overall view, and with statistical significance (Table 2 and Figure 5).
Physical therapy (electromagnetic fields and manual therapy) plus TIOBEC® (α-lipoic acid plus vitamin B, C, and E) improved all outcome measures (pain, edema, and function) in an overall view, in patients with complex regional pain syndrome. CRPS, Complex regional pain syndrome; VAS, Visual Analogue scale.
5. Discussion
As far as the authors are concerned, this is the very first article that states the effectiveness of physical therapy (electromagnetic fields and manual therapy) plus TIOBEC® (α-lipoic acid plus vitamin B, C, and E) in the management of pain and edema in a small series of CRPS.
CRPS is a common and devastating complication that occurs after trauma and immobilization and is very frequent after wrist fractures (1% to up to 37%) and after volar locking plate fixation (3% to up to 10%) (11, 13). CRPS development considerably lengthens the time of recovery after a traumatic injury, causing a great impact on work, social activities and psychological well-being (13). It is reported that CRPS has an estimated prevalence rate of 21/100,000; that work absenteeism is substantial and the financial impact on the health care system is also considerable (11). Since CRPS is a chronic and disabling disease, to prevent its appearance is of paramount importance (11).
CRPS is an overestimated inflammatory state secondary to a traumatic injury that activates the sympathetic nervous system producing pain, edema, and limitation in the arch of motion (13). Therefore, the main goals of treatment are to act over inflammation by the use of electromagnetic fields and TIOBEC® and to act over rigidity by the use of manual therapy, as it was observed in the present study.
Electromagnetic fields (EMF) is a physical therapy that has the capability to inhibit inflammation and to modulate pain (15). EMF inhibits presynaptic pain fibers and decreases their excitability (15). EMF decrease membrane polarization level to -90 mV; therefore, the transmission of pain signals is blocked (15). EMF restores cellular and functional integrity at the biological level. EMF improves oxygen delivery, vasodilatation of blood vessels and pain reduction. EMF reduces edema and pain secondary to a traumatism to soft tissue (15). For those reasons, we have considered EMF for the protocol of the present study.
Physical therapy and manual therapy have demonstrated to be capable of reducing pain and to improve range of motion in patients diagnosed with CRPS of the upper limbs (19). Patients in our study reduced pain and improve range of motion from an overall point of view.
Diverse and updated studies have stated that vitamin C and α-lipoic acid (ALA) have shown an anti-inflammatory effect and therefore may exert a biological effect on CRPS (11-13). Therefore we decided to use as adjuvant therapy TIOBEC®, which is a combination of vitamin B, C, E and α-lipoic acid for the management of pain and inflammation in CRPS, a fact that has been clearly demonstrated in our present study.
In the case of α-lipoic acid, Boriani has stated that α-lipoic acid has a potent anti-oxidant effect and it is also able to decrease axonal sensitivity on pain by inhibition of Ca+ channels of the neuronal T-type (12). Pajardi et al. (20) have reported that α-lipoic acid with curcumin and B group vitamins administered for three months (90 days) reduced nocturnal pain in patients with carpal tunnel syndrome. Alpha-lipoic acid protects nerves from lipid peroxidation and ischemia improves nerve conduction of distal motor and sensory nerves and increases nerve blood flow to such structures (12).
In the case of vitamin C, two randomized studies have stated that a daily dose of 500 mg of vitamin C reduced the prevalence of CRPS after wrist fracture (14). The rationale to use vitamin C is that this vitamin inhibits inflammatory pathways because of their antioxidant properties (21). Vitamin C is an important reducing agent that binds harmful free radicals, which are usually released on inflammation processes (14). Zollinger et al. (22) has reported that the administration of prophylactic vitamin C administration in patients with wrist fractures decreased the prevalence of CRPS to 7% if compared to 22% in the placebo group. In the previous study, if surgery treated patients were considered, the prevalence of CRPS was 2.4%, if compared to 10% in the placebo group. There is a clear demonstration that vitamin C has a role in the management of CRPS and its prevention.
In our study, a combination of α-lipoic acid (800 mg) and vitamin C (60 mg) was used for the management of CRPS, in the form of TIOBEC® formulation. Although the dosage of vitamin C was inferior to the previous studies (500 mg), we believe that the combination of both anti-inflammatory compounds (α-lipoic acid and vitamin C) may potentiate each other and therefore, the desired anti-inflammatory effect was clearly observed in our study.
One important limitation of the study was the small sample size. However, the results represent real clinical management of CRPS in our rehabilitation setting. The larger sample size is needed to corroborate our promising results.
5.1. Conclusions
Complex regional pain syndrome is a devastating and disabling complication that lengthens the recovery time for patients. The present study suggests that the protocol of rehabilitation and physical therapy that includes the electromagnetic field, manual therapy and TIOBEC® (α-lipoic acid plus vitamin B, C, and E) may be safely used in patients with CRPS in an attempt to ameliorate pain, to reduce edema and to improve function. In light of the results of this study, the protocol used might accelerate recovery time. More studies are needed to corroborate the present results observed in this pilot study.