1. Background
The brain-derived neurotrophic factor (BDNF) is a protein that promotes retention through growth, differentiation, and protection of nerve cells. This protein is associated with growth, synaptic plasticity, learning, and facilitation of cognitive processes (1). Different studies have tried to demonstrate its role in the efficiency of cognitive functions by regulating the expression of BDNF in various ways. In these studies, the relationship between fear learning and increased BDNF and tropomyosin receptor kinase B (TrkB) expression in rats’ amygdala was investigated (2). These studies showed gradual increased expression of BDNF in the motor cortex due to the learning of a skilled forelimb reaching task by rats (3), improved spatial and non-spatial learning induced by intracerebroventricular injection of the BDNF protein (dentate gyrus, hippocampus, and perirhinal cortex) in a group of rats, and its increase over a week of running on a treadmill in another group (4), and increased memory performance along with BDNF induced by intense aerobic exercise in the rat’s brain (5).
A study revealed that increasing BDNF concentration in human serum (due to exercise) was accompanied by improvement in the performance of the face-name matching task and the Stroop color-word task (6). Also, in a study by Egan et al. (1), decreased levels of BDNF in the human brain were associated with cognitive deficits and impaired memory. In another study on women, BDNF was also associated with memory performance and memory circuity function (7).
Studies suggest that a single nucleotide polymorphism (SNP) at codon 66 of the BDNF gene of some individuals, located on chromosome 11, results in an amino acid substitution (valine to methionine) in one or both alleles available in this region. This event leads to three types of genotypes: those with two valines (Val/Val), those with a valine and methionine (Val/met), and people with two methionines (met/met). Individuals carrying two valines or two methionines are called homozygote, and people carrying a valine and methionine are called heterozygote (1). In different societies, there are certain proportions of these genotypes. For example, in Germany, this ratio is 60% for Val/Val and 40% for met-carrier (met/met and Val/met) (8).
This polymorphism does not alter the structure of the adult BDNF but disrupts its amount and severity of expression. Val66met polymorphism is also associated with a decrease in the release of activity-dependent BDNF (1). In previous studies, these defects have been associated with behavioral and neuroanatomical differences among young people. For example, hippocampal volume (9) and its function and episodic memory (1, 10) in met-carriers (in the rest of the text, instead of using this term, the term “people with the polymorphism or met-carriers” is used) are reduced.
Given the expression of BDNF in several cerebral structures, including the cerebral cortex, it is tempting to assume that the polymorphism may affect learning, memory, and various cognitive functions. For example, Joundi et al. (11) showed that met-carriers were significantly weaker than people without the polymorphism in the learning of visuomotor adaptation task. In other studies utilizing fMRI and non-invasive electrical and magnetic stimulation of the brain, it has been suggested that val66met polymorphism is associated with short-term plasticity of the motor cortex (12, 13). Also, Hariri et al. (14), using the BOLD FMRI, showed that met-carriers had a weaker hippocampal activation than people without the polymorphism during encoding and retrieval processes. In this study, met-carriers were also weaker in the declarative memory task. In 2005, Eker et al. (15), by examining the gray matter of various brain regions, concluded that the BDNF vl66met polymorphism had a significant adverse effect on brain structures involved in the working memory network. However, there have also been studies that have not been able to repeat this finding with relatively different protocols (16). Due to the different effects on motor behavior and especially motor learning, some studies have shown the effects of different BDNF genotypes on short- and long-term learning, while others have failed to show such effects (17).
In a study by Tonacci et al. (18), it was shown that met-carriers had disturbances in olfactory functions. Defects in these functions are a significant contributor to neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. Moreover, in patients with a bipolar mood disorder, met-carriers poorly perform the Wisconsin card sorting task, which is related to the prefrontal lobe (19). However, Freundlieb et al. (17) showed that there was no difference between met-carriers and people without polymorphism in short-term implicit learning patterns (motor learning and vocabulary learning. Interestingly, Beste et al. (20) revealed that met-carriers are more able to do response inhibition than those without the polymorphism. On the other hand, some studies have revealed apparent differences in the prevalence of types of BDNF genotype and their behavioral effects in different races (21, 22).
Among cognitive functions, attention is of paramount importance. The ability to pay attention makes it possible for humans to control the input of different stimuli into the conscious scene of the mind and choose only a few among various stimuli. The power to maintain attention is one of the most prominent characteristics of rational growth that is impaired by the lack of nervous system growth (23). On the other hand, attention is associated with other cognitive functions, including learning. Attention capabilities are a pre-requisite for learning. A study has shown that selective attention is an essential factor in motor learning because paying attention to the relevant stimulus and ignoring irrelevant stimuli is one of the basic principles of observational learning (24).
2. Objectives
Therefore, considering the inconsistency in the results of previous studies, the difference in the prevalence and behavioral effects of different types of BDNF genotype in different ethnic groups, and the fundamental role of attention and motor learning in everyday life, we attempted to investigate whether the presence of val66met polymorphism in some people in an Iranian population differentiates them from the rest of the population in terms of cognitive capabilities.
3. Methods
3.1. Subjects
The study was conducted among 100 Iranian students from the University of Kashan with the mean age of 21.60 ± 2.20 years and age range between 19 to 25 years old. They all were informed of the research procedures, and written informed consent was obtained from them. There was no history of serious medical, neurological, psychiatric, behavioral, and motor problems among the participants. They had never used illegal, neuroactive, or recreational drugs (> 15 cigarettes/day, > 6 cups of coffee/day, > 50 g of alcohol/day) (17). Considering the possible effect of sex hormones on regulating the expression of BDNF (25, 26), among all the participants, only single men were selected. The experiments reported in this study were performed under the ethical standards of Kashan University of Medical Sciences and Health Services.
3.2. BDNF Genotyping
BDNF genotyping was performed as described in Nooshabadi et al. study. Based on the analysis, the students were divided into two groups of Val/Val (46 people) and met-carriers (54 people). The genotype distributions of Val66Met polymorphism observed in both Val/Val and Val/ met genotype satisfied the Hardy-Weinberg equilibrium criterion and were comparable with that previously observed in previous studies (8, 18, 20). The principal researcher and all the participants were blinded to the genotype of the participants.
3.3. Stroop Color-Word Test
Stroop test was used to measure attention. This test was first devised by Ridley Stroop in 1935 to measure selective attention and cognitive flexibility (27). In this test, the interval of the stimulus is 800 milliseconds, and the duration of each stimulus is 2000 milliseconds. This test should be carried out in a quiet and suitable place, and the terms of running the test should be considered in terms of psychometry. Research on this test indicates the appropriate validity and reliability of assessment in adults (28) and children (29). This test’s test-retest reliability has been reported to range from 0.80 to 0.91 (29).
After entering the personal information of each person in the relevant part, the researcher by displaying the monitor to the participant declares that: a picture of red, yellow, green and blue colors is consecutively shown on the computer monitor, and the participant must specify the correct color at the highest speed by clicking the specified keys. After performing this section, to get familiar with running this test, in the main part of the test, the person is told that he/she will see colored words that should be answered only on the correct color. Color-name words may have different colors (for instance, the word “blue” is printed in red). In this step, 48 congruous color words (the color of the word is identical with the word meaning) and 48 incongruous color words (the color of the word is not matched with the word meaning), that is, a total of 96 congruous and incongruous color words are randomly and consecutively provided to the participant in just one phase. The participant has to determine the only correct color. The software calculates the reaction time of the individual in responding to each word (congruous and incongruous) and the number of correct or incorrect answers. The participant’s score includes the intervention time and the intervention score, which is calculated by subtracting the sum of the time and the number of correct answers of the congruous attempts from the sum of the time and the number of correct answers to the incongruous attempts (30).
3.4. Serial Reaction Time Test
The Serial Reaction Time test (SRTT) is a choice reaction time task carried out with four different fingers in eight blocks, as described in Freundlieb et al. (17). A decrease in reaction time is considered as an improvement of general visuomotor performance, the difference between sequential and random block response times is regarded as a measure for implicit motor learning (IML).
3.5. Design and Procedure
Having determined the individuals’ genotype, they were divided into two groups with and without the polymorphism. The Stroop color-word test was then performed one day, and afterward, the serial reaction time test was performed on another day under the same conditions for all the participants in a completely quiet place with appropriate lighting and conditioning.
3.6. Statistical Analysis
The Kolmogorov-Smirnov test was used to determine the normality of the distribution of data. Also, independent t-test and ANCOVA tests were run to test differences between the groups. For all the statistical analyses, a P value of less than 0.05 was considered statistically significant.
4. Results
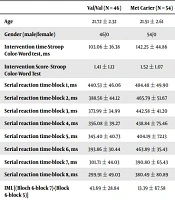
Demographic and descriptive data are summarized in Table 1.
| Val/Val (N = 46) | Met Carier (N = 54) | |
|---|---|---|
| Age | 21.72 ± 2.32 | 21.51 ± 2.61 |
| Gender (male/female) | 46/0 | 54/0 |
| Intervention time-Stroop Color-Word test, ms | 103.06 ± 36.38 | 142.25 ± 44.86 |
| Intervention Score- Stroop Color-Word Test | 1.41 ± 1.13 | 1.52 ± 1.07 |
| Serial reaction time-block 1, ms | 440.53 ± 46.06 | 484.48 ± 49.90 |
| Serial reaction time-block 2, ms | 388.56 ± 44.12 | 465.79 ± 51.67 |
| Serial reaction time-block 3, ms | 373.99 ± 34.99 | 442.58 ± 41.20 |
| Serial reaction time-block 4, ms | 356.08 ± 39.27 | 438.84 ± 75.46 |
| Serial reaction time-block 5, ms | 345.40 ± 40.73 | 404.19 ± 72.13 |
| Serial reaction time-block 6, ms | 393.86 ± 30.44 | 463.89 ± 35.43 |
| Serial reaction time-block 7, ms | 301.71 ± 44.03 | 390.80 ± 65.43 |
| Serial reaction time-block 8, ms | 299.91 ± 49.03 | 380.49 ± 80.89 |
| IML [(Bloch 6-block 7)-(Block 6-block 5)] | 43.69 ± 28.84 | 13.39 ± 67.58 |
aValues are expressed as mean ± SD.
Independent t-test showed a significant difference between the groups in the intervention time of the Stroop test (t = 4.696, P = 0.001) and implicit motor learning of the Serial Reaction test (t = 2.801, P = 0.006). However, no significant difference was seen between the groups in the intervention score of the Stroop test (t = 0.474, P = 0.637) (Table 2).
Analysis of covariance showed a significant difference between the groups at Block 8 of Serial Reaction time (F = 11.674, P = 0.001) (Table 3).
5. Discussion
Using the serial reaction time test, the differences between people without val66met polymorphism and met-carriers in terms of both visuomotor performance and implicit motor sequence learning were investigated. The results of this test showed that people without the polymorphism were stronger than met-carriers in visuomotor performance (which is determined through the review of the progress of the groups and the difference between groups in block (8). Also, between people without val66met polymorphism had a higher ability than met-carriers to learn implicit motor sequences (through the examination of the score IML). Therefore, it can be concluded that the polymorphism affects these capabilities. Also, using the Stroop test, it was found that met-carriers had a higher intervention time in responding to the stimuli than people without the polymorphism, although they did not differ significantly in terms of intervention score. This result suggests that met-carriers are weaker than people without polymorphism in terms of selective attention.
BDNF plays an important role in neuronal protection and neurogenesis. On the one hand, several studies have proven that this protein plays an important role in the synaptic plasticity of the hippocampus, and on the other hand, the hippocampus is useful in learning, memory, and cognitive functions (6). It has been reported in these studies that any factor that increases human BDNF levels can change some cognitive functions such as fear learning (2), spatial and non-spatial learning, memory (4), and matching tasks (4). Regarding these interpretations, it seems rational that the factor that has caused the disruption of the secretion and expression of this protein in some individuals (i.e., val66met polymorphism) can challenge these individuals’ capabilities in the implementation of the serial reaction time test and the Stroop Color-Word test.
The results of this study are consistent with those of the most recent studies. Hariri et al. (14) used BOLD fMRI to investigate the relationship between BDNF genotype and hippocampal activity during implicit memory processing. In the study, it was shown that met-carriers, during encoding and retrieval processes, have lower hippocampal activation than those without polymorphism. Also, in this study, met-carriers were weaker in the declarative memory function.
Joundi et al. (11) also demonstrated that met-carriers were significantly weaker than people without the polymorphism in the learning of visuomotor adaptation task. Also, Rybakowski et al. (19) showed that people without polymorphism performed weaker in the Wisconsin card sorting task than met-carriers. The researchers attributed the results of their research to the possible role of BDNF in prefrontal cortical structures.
However, the results of the present study are inconsistent with the results of the study by Freundlieb et al. (17). In that research, no difference was found between the two groups in serial reaction time test and the vocabulary learning task, both of which are short-term implicit learning patterns. The contradiction between the results of this study and the research by Freundlieb et al. (17) may be due to the difference between the number of participants in the two studies and the lack of gender-control in the study of Freundlieb et al. (17) It is shown that estrogen, which is a female sex hormone, is a stimulant of BDNF expression (25); thus, Freundlieb et al. (17) in their study concluded that the disturbance caused by the val66met polymorphism is likely to be incorrect.
The results of this study are also inconsistent with those of Beste et al. (20). In that study, a contradictory result compared to most studies in this area was obtained. They showed that met-carriers had a better response inhibition than those without the polymorphism. They attributed this finding to the fact that the basal ganglia circuits control the ability to inhibit the response, and impairment of BDNF expression results in a decrease in the activity of the nigro-estratital in the basal ganglia. This reduction, although reducing the activity of the direct pathway of the basal ganglia, increases the activity of an indirect pathway within the basal ganglia, leading to a change in the dominant pathway in met-carriers (20). Besides, in that study, similar to the study by Freundlieb et al. (17), the participants were not gender-controlled, which could have caused the difference between the results of that research and the present results.
One of the most important limitations of this study was the lack of gender control. Considering the possible effect of sex hormones on regulating the expression of BDNF, only men were selected. Thus, we suggest future studies investigating the effect of gender on the polymorphism or the effect of the polymorphism on women.
5.1. Conclusions
In general, the results of this study showed that val66met polymorphism affects visuomotor task, implicit motor sequence learning, and selective attention, such that the presence of this polymorphism in some individuals is likely to weaken their ability through disrupting BDNF secretion compared to people without the polymorphism. This result can be remarkable because the present study has been conducted in a population different from those of previous studies, and considering the research literature, there is a possibility of a difference in the prevalence and magnitude of the polymorphism effect at various populations. Nonetheless, in order to extrapolate the present results to other populations, further research is warranted.