1. Background
Stroke is a sudden disastrous event affecting all aspects of an individual’s life. Stroke is the number one cause of adult disability worldwide, and most people who survive this occurrence experience a variety of disabilities to varying degrees (1). After a stroke, survivors often experience emotional and social changes in their life due to these disabilities, and feelings of anger, anxiety, or depression and social isolation are common (2).
Oropharyngeal dysphagia (OPD) is a common disability in stroke survivors (3). The prevalence of OPD has reported in different studies between 14% and-94 percent (4). Swallowing disorder in stroke patients has medical, psychosocial, and economic consequences. Medical complications include malnutrition, dehydration, aspiration pneumonia, and even death (5). Other complications of dysphagia in stroke survivors include psychosocial complications because swallowing is part of the eating process and eating as a daily activity that is essential for health also is a pleasurable and psychosocial activity (6). People usually eat together but many dysphagia patients avoid eating with others during mealtimes due to anxiety, fear of choking, and the use of new methods for eating (7). Dysphagia patients for safe and effective swallowing may use new methods for eating, and these changes in eating habits lead to depression and social isolation in these patients and all of this leads to dissatisfaction with the patients (8). In a review study, Davis found that dysphagia, like other chronic conditions, had a negative effect on patients’ quality of life (9). According to various studies, health professionals should systematically consider the perception of stroke survivors concerning their health status and quality of life in clinical assessments and interventions (1, 10).
Speech-language pathologists as one of the main members of the stroke management team should consider all areas affected by dysphagia including physical, spiritual, emotional, nutritional, and social in addition to comprehensive clinical and instrumental dysphagia assessments and providing treatment methods to improve swallowing disorders and quality of life in stroke survivors (11, 12).
Quality of life as a multidimensional concept is completely individual and cannot be observed by others and is based on an individual’s perception of various aspects of their lives in the cultural context (12). However, there are still few studies about the effect of a type of disability on the change of quality of life (QOL) of stroke survivors, and studies have examined these effects in general (13). Various studies have also shown that different factors including age, gender, dependency in activities of daily living/disability, social support, depression, affect the quality of life of stroke survivors (14-17). But these studies have not reached a definite conclusion, and even their results are conflicting in part, because the quality of life is a multidimensional and complex concept.
Therefore, due to the high prevalence of dysphagia in stroke and its psychological and social consequences for survivors, as well as the lack of a study in Iran that examines the role of this disability and its factors affecting the quality of life of stroke patients, it is necessary to conduct studies in this field.
2. Objectives
This study aimed to investigate the impacts of dysphagia on quality of life in Iranian stroke survivors and to determine potential relationships between demographic variables and the domains of quality of life.
3. Methods
3.1. Study design and Participants
This study was approved by the Research Ethics Committee of Semnan University of Medical Sciences, Semnan, Iran (Reference Number. IR.SEMUMS.REC.1395.64). Before any examination, the informed consent form was completed by the patients to participate in this study and all participants have referred for swallowing rehabilitation to speech therapy clinics.
Sixty stroke survivors with a diagnosis of oropharyngeal dysphagia were recruited from neurology wards of Tehran and Semnan hospitals and Outpatient rehabilitation centers of Tehran and Semnan cities and participated in this cross-sectional study. Inclusion criteria include age range 60 - 75 years, diagnosis of stroke which had done by the neurologist, presence of oropharyngeal dysphagia on the base of the Mann Assessment of Swallowing Ability (MASA), no history of other neurological diseases affecting swallowing function (Parkinson’s disease, Multiple sclerosis), no history of chronic pulmonary disease, At least two weeks after the stroke, Ability to read and write in Persian, Relatively good auditory comprehension on the base of subtest of auditory comprehension of Persian aphasia test.
Swallowing ability was assessed by the Mann Assessment of Swallowing Ability (18). After diagnosing oropharyngeal dysphagia in patients, the patient’s quality of life was examined by the Persian version of the Dysphagia Handicap Index (19).
3.2. Instruments
3.2.1. Mann Assessment of Swallowing Ability
The MASA was first developed by Dr. Gisele Mann and her colleagues in 2002 to diagnose dysphagia in stroke patients, and its sensitivity (71%) and specificity (72%) were identified. This test consists of 24 items. The total scores obtained from this test are 200 and its cut-off point is 177. The score above 178 is interpreted as normal swallowing, 168–177 as mild dysphagia, 139–167 as moderate dysphagia, and less than138 as severe dysphagia (18).
3.2.2. Persian-Dysphagia Handicap Index (P-DHI)
The Dysphagia Handicap Index (DHI) is a self-reporting tool related to the effect of dysphagia on patients’ quality of life. It consists of 25 items in three subsections: physical (9 items), functional (9 items), and emotional (7 items). There are three options for each item that scored zero for never, two for sometimes, and four for always. The range of scores is between 0-100 and a higher score indicates a greater negative impact of dysphagia on patients’ quality of life. Also, DHI has a self-reported severity of Dysphagia that the patient is asked to rate their dysphagia severity from one to seven. The severity of dysphagia is defined as follows: 1 for no dysphagia (normal), 2 and 3 for mild, 4, and 5 for moderate and 6 and 7for severe. The (P-DHI) has good validity and the test-retest reliability for the total and three subscales is between 0.95 and 0.98 (19).
3.3. Statistical Analysis
In the present study, continuous variables were expressed as mean and standard deviation (SD) and categorical variables as frequency (percentage). The inspection of the normal distribution was done with the Shapiro-Wilk test. Demographic characteristics between groups were compared using the Mann-Whitney U test. Besides, the Pearson correlation coefficient and Spearman’s correlation coefficient were conducted to examine the relationship between DHI total scores and demographic/clinical variables. Furthermore, Partial Correlation was used to describe the relationship between two variables (severity of dysphagia and DHI total scores) whilst taking away the effects of another variable (age and education level). Data analysis was undertaken using IBM SPSS Statistics for Windows, version 24 (SPSS Inc., Chicago, IL, USA), and P-value less than 0.05 was considered statistically significant.
4. Results
4.1. Participants’ Characteristics
Table 1 outlines the demographic and clinical characteristics of the 60 stroke survivors (68.3% men). The mean age and disease duration of the patients were 67.08 ± 2.8 and 5.60 ± 3.05 weeks, respectively, and 20% of patients had an academic education.
| Characteristics | Frequency (%) | Min - Max | Mean | SD | P Value |
|---|---|---|---|---|---|
| Age, y | - | 62 - 75 | 67.08 | 2.80 | 0.022 |
| Gender | - | < 0.001 | |||
| Male | 41 (68.3) | ||||
| Female | 19 (31.7) | ||||
| Education | < 0.001 | ||||
| Primary | 15 (25) | ||||
| Secondary | 33 (55) | ||||
| University | 12 (20) | ||||
| Time post-onset of stroke, wk | - | 2 - 12 | 5.60 | 3.05 | < 0.001 |
| MASA Scores | - | 130 - 175 | 149.63 | 13.71 | < 0.001 |
| DHI Scores | - | 56 - 86 | 73.03 | 10.16 | < 0.001 |
Demographic and Clinical Characteristics of Participants
The mean of MASA scores was 149.63 ± 13.71 which indicates the severity of dysphagia among participants in this study was moderate. The mean of DHI scores was 73.03 ± 10.16.
4.2. Bivariate Analysis
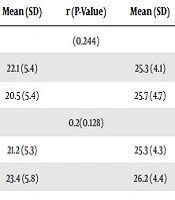
According to the Pearson correlation coefficients, DHI total score was not correlated with age (r = 0.203, P = 0.121) ,as well as its subscales did not indicate this correlation (P > 0.05). Also, DHI total scores was not correlated with time post-onset of stroke (r = 0.097, P = 0.459). Also there was no significant difference in DHI total score among men and women (P = 0.778), and the scores of subscales of DHI did not differ significantly between men and women (P > 0.05). In stroke survivors, education level was positively correlated with the functional subscale of DHI (r = 0.270, P = 0.037) (Table 2).
| Characteristics | No. (%) | DHI Total and Subscales Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Emotional | Physical | Functional | DHI Total Score | ||||||
| Mean (SD) | r (P-Value) | Mean (SD) | r (P-Value) | Mean (SD) | r (P-Value) | Mean (SD) | r (P-Value) | ||
| Gender | (0.244) | (0.712) | (0.309) | (0.778) | |||||
| Male | 41 (68.3) | 22.1 (5.4) | 25.3 (4.1) | 25.2 (4.4) | 73.3 (10.2) | ||||
| Female | 19 (31.7) | 20.5 (5.4) | 25.7 (4.7) | 26.3 (4.3) | 72.5 (10.3) | ||||
| Age, y | 0.2(0.128) | 0.18 (0.182) | -0.03 (0.852) | 0.20 (0.121) | |||||
| < 70 | 50 (83.3) | 21.2 (5.3) | 25.3 (4.3) | 25.6 (4.4) | 72.5 (10.4) | ||||
| ≥ 70 | 10 (16.7) | 23.4 (5.8) | 26.2 (4.4) | 25.0 (4.2) | 75.6 (9.2) | ||||
| Stroke duration, wk | 0.09 (0.516) | 0.02 (0.877) | 0.14 (0.291) | 0.09 (0.459) | |||||
| < 5 | 33 (55) | 20.6 (5.5) | 25.1 (4.3) | 24.7 (4.2) | 71.0 (10.6) | ||||
| ≥ 5 | 27 (45) | 22.7 (5.2) | 25.8 (4.3) | 26.6 (4.4) | 756 (9.2) | ||||
| Educational level | 0.20 (0.120) | 0.11 (0.419) | 0.27 (0.037) | -0.05 (0.692) | |||||
| Primary | 15 (25) | 22.1 (5.6) | 26.7 (4.6) | 22.9 (4.1) | 71.7 (10.8) | ||||
| Secondary | 33 (55) | 21.9 (5.6) | 24.4 (4.1) | 26.1 (4.0) | 73.3 (10.3) | ||||
| University | 12 (20) | 19.8 (4.8) | 26.7 (3.7) | 27.3 (4.5) | 73.8 (9.7) | ||||
| Dysphagia severity | 0.24 (0.062) | 0.16 (0.238) | 0.27 (0.039) | 0.22 (0.089) | |||||
| Mild | - | - | - | - | - | ||||
| Moderate | 25 (41.7) | 20.3 (5.6) | 25.0 (4.2) | 24.2 (4.2) | 70.3 (10.7) | ||||
| Severe | 35 (58.3) | 22.5 (5.1) | 25.8 (4.3) | 26.5 (4.3) | 75.0 (9.4) | ||||
Relationship Between DHI Total Score and Its Subscales Scores with Demographic/Clinical Characteristics in Stroke Survivors with Dysphagia
Besides, the severity of dysphagia on the base of MASA scores was positively correlated with the functional subscale of DHI (r = 0.267, P = 0.039) whereas these relationships were not observed between the severity of dysphagia and emotional (P = 0.062) and Physical (P = 0.238) subscales of DHI (Table 2).
According to the Partial correlation, by controlling for the effect of age and education level, there was relationship between severity dysphagia and DHI total score (r = 0.312, P = 0.017) as well as emotional subscale (r = 0.262, P = 0.047) and functional subscale (r = 0.323, P = 0.013) (Table 2).
5. Discussion
Disabilities following a stroke lead to a decline in the quality of life in stroke survivors. The results of this study indicate that dysphagia as a common disability in stroke survivors reduces the quality of life of these persons and more severe dysphagia has a more negative impact on QOL. These results are consistent with previous studies that dysphagia can cause a decline in the QOL of patients (20, 21).
Another finding of this study was the relationship between education level and the functional aspect of quality of life in stroke survivors, while there was no correlation between age, gender, and duration after stroke and quality of life. But contrary to these findings, Hackett et al. in their studies have shown that age is negatively related to the quality of life in stroke survivors witch this difference is probably due to the difference in the sample size of the study, so that the sample size of our study was small, while in these studies the sample size was very large (22). Also, the diversity of participants in our study was low. But, the effects of gender on quality of life in this study are consistent with previous studies, and the effect is similar for survivors of both genders (23).
The relationship between education level and quality of life has been confirmed in other studies and it has been shown that people with higher education will have a higher quality of life. This is because higher education develops basic cognitive functioning and it increases problem-solving skills to adapt to problems (24, 25).
Another finding of this study, which is not consistent with other studies (26-28), is that there is no correlation between the duration of stroke and quality of life. While other studies have reported that when the duration of the disease is prolonged, its negative impact on patients’ quality of life will be greater. This difference is because rehabilitation interventions play an important role in improving the disability of stroke patients.
Contrary to studies that have examined the quality of life in patients with dysphagia with generic measures (29), or administered questionnaires that assess the overall quality of life, in this study we used a dysphagia handicap index (DHI) (30) to examine the effect of dysphagia on the quality of life of stroke patients. This index measures the handicapping effect of dysphagia on aspects of an individual’s lives such as emotional, functional, and physical. Besides, this index is a patient-reported outcomes tool for dysphagia that it’s focusing on the patient experience of having dysphagia, combined with their medical diagnosis, provides a broad, meaningful picture of the health of an individual and can assist health professionals in the decision-making process of management of stroke.
The main goal of rehabilitation in stroke patients is to maximize their independency for activity daily living and increase their quality of life. Thus, Speech and language pathologists need to evaluate different domains of quality of life in addition to clinical and instrumental assessments of swallowing function in stroke patients. The addition of a quantitative measure of patient self-assessment of dysphagia will.
Quality of life is an important indicator of the outcome after a stroke and can improve our clinical decisions making and is an objective indicator for determining the effectiveness and efficiency of dysphagia treatment in stroke survivors.
5.1. Limitations
This study has some limitations. First, the sample size of this study is small, which may cause some bias and a larger sample size may allow a greater variety of responses for dysphagia severity. Secondly, there was no control group to compare the quality of life between stroke survivors with dysphagia and without dysphagia.
5.2. Conclusion
Based on the findings of this study, it can be concluded that dysphagia in stroke survivors has negative impacts on the quality of life. The degrees of dysphagia have the greatest impact on emotional and functional aspects of quality of life in stroke survivors. Dysphagia’s negative impact on the quality of life does not depend on age and gender.