1. Background
The imbalance is the most important complication after a stroke (1). Reduced muscle strength, decreased range of motion, poor motor coordination, and sensory system disorders can cause imbalance (1). Changing the position from sitting to standing is one of the essential activities of daily life (2), which is the basis of transitional activities from bed to the chair and chair to the toilet (3). These are essential for walking and mobility and require the acceptable ability of lower limb function and postural control (4). Stroke patients transferred less weight to their affected leg than unaffected one during sit-to-stand positions, which makes them unable to change their positions (5). It has been reported that placing the affected foot behind the unaffected foot reduces the asymmetry in response to vertical forces during changing position; thus, assessment and restoration of suitable and symmetrical postural stability are one of the important goals to prevent these patients from falling into the process of rehabilitation (5). The timed up and go (TUG) test is the basic test used to evaluate motor function and the ability to maintain balance (6). This test is performed quicker than other tests and has good sensitivity and validity (6). The initial placement of foots appears to affect the time of the TUG test so that when the affected foot is placed behind the unaffected one, the duration of the TUG test is longer than other strategies (5).
Transcranial direct current stimulation (tDCS) is a non-invasive treatment that modulates brain tissues (7). It has been shown that the application of tDCS, along with peripheral electrical stimulations, modulates inefficient network activities and, by affecting the relevant routes, develops a compromise balance in the disrupted network for appropriate outputs and suppresses incompatible changes (8). Various studies have been performed to assess the effect of tDCS on the balance, gait, and function of stroke patients (9-11). In a meta-analysis study, Kang et al. showed that postural control and balance of the stroke patients improved by stimulating the brain (10). The use of tDCS in the secondary motor area of the brain, along with bodyweight support during gait training on the treadmill, is also reported to be helpful in improving postural control in these patients (9). Therefore, the effectiveness of the gait training with repeated tDCS has been proven (12). The application of tDCS has also been shown to improve movement and muscle strength in stroke patients (13). Another study found that the use of tDCS over the primary motor cortex in children with cerebral palsy boosts motor patterns and improved balance and gait. This study showed that the use of a single session anodal tDCS has made changes in the excitability of the primary motor cortex in children with cerebral palsy and could improve the motor pattern by increasing the excitability of the cerebral cortex and activating the corticospinal pathway (14). Researchers believe that facilitating the excitability of the primary motor cortex may increase motor control and speed of motor responses (14).
The cerebellum is known to be involved in error-based motor learning and motor adaptation (15). Long term depression-like plasticity of Purkinje cells is associated with learning. This process is mediated by simultaneous activation of parallel fibers and climbing fibers that give input to error signals in motor control to the cortex (16). Balance performance can be seen as an adaptation of the posture (17), and the cerebellar hemispheres play an important role in motor adaptation (18, 19). The connection of the M1–cerebellar also results in more accurate movement endpoints, emphasizing the specific role of the cerebellum in motor adaptation (15, 17, 20). The medial flocculonodular lobe of the cerebellum is directly linked to postural balance (21). Therefore, cerebellar tDCS is a simple physiological tool that is useful in patients with cerebellar dysfunction or psychiatric disorders and those undergoing neurorehabilitation to enhance neuroplasticity (22).
Applying tDCS before routine physiotherapy is common for improving standing posture (23), and studies have also shown that it is effective in improving the balance of stroke patients (14, 23).
2. Objectives
Owing to foot position during sit-to-stand transition, it has been reported that placing the affected foot behind the unaffected foot increases the duration of the TUG test. The purpose of the present study was to investigate the effect of cerebellar tDCS on the TUG test with different foot positions in chronic stroke patients.
3. Methods
3.1. Participants
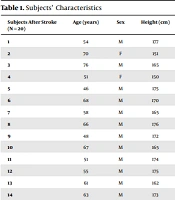
Twenty subjects were recruited from university clinics from March to July 2018 (Table 1). The participants recruited for this study were community-dwelling stroke survivors who met the inclusion criteria of chronic (> 6 months) stroke (24), with good cognition, and were able to walk with (i.e., cane) or without a walking aid (24). Exclusion criteria were subjects with aphasia, incontinence, and history of falls (25). The Ethical Committee of Semnan University of Medical Sciences approved the study protocol (IR.SEMUMS.REC.1396.167), and all subjects provided written informed consent. The study was registered as a clinical trial study on the Iranian Registry of Clinical Trials (IRCT20160424027562N5).
| Subjects After Stroke (N = 20) | Age (years) | Sex | Height (cm) | Weight (kg) | BMI | Time After Stroke, (months) | FMA-LE (Max = 34) | Walking Aid | Paretic Side | MAS PF (Max = 5) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | 177 | 78 | 24.90 | 7 | 23 | None | R | 2 |
| 2 | 70 | F | 151 | 70 | 30.70 | 224 | 26 | Cane | L | 3 |
| 3 | 76 | M | 165 | 80 | 29.38 | 7 | 27 | None | R | 0 |
| 4 | 51 | F | 150 | 80 | 35.56 | 9 | 27 | None | R | 3 |
| 5 | 46 | M | 175 | 97 | 31.67 | 36 | 32 | None | R | 1 |
| 6 | 68 | M | 170 | 70 | 24.22 | 7 | 27 | None | L | 0 |
| 7 | 58 | M | 165 | 70 | 25.71 | 36 | 29 | None | R | 0 |
| 8 | 66 | M | 176 | 81 | 26.15 | 48 | 29 | None | R | 0 |
| 9 | 48 | M | 172 | 74 | 25.01 | 36 | 26 | None | R | 2 |
| 10 | 67 | M | 165 | 86 | 31.59 | 72 | 31 | None | L | 1 |
| 11 | 51 | M | 174 | 65 | 21.47 | 12 | 17 | None | L | 3 |
| 12 | 55 | M | 175 | 84 | 27.43 | 48 | 29 | None | R | 1 |
| 13 | 61 | M | 162 | 86 | 32.77 | 10 | 18 | None | L | 2 |
| 14 | 63 | M | 173 | 69 | 23.05 | 24 | 32 | None | R | 0 |
| 15 | 36 | M | 177 | 88 | 28.09 | 6 | 30 | None | R | 0 |
| 16 | 59 | M | 164 | 74 | 27.51 | 18 | 28 | None | R | 0 |
| 17 | 75 | M | 157 | 65 | 26.37 | 8 | 32 | None | R | 1 |
| 18 | 60 | F | 152 | 93 | 40.25 | 24 | 30 | None | L | 2 |
| 19 | 79 | M | 164 | 81 | 30.12 | 36 | 29 | Cane | R | 1 |
| 20 | 64 | M | 150 | 77 | 34.22 | 26 | 13 | None | R | 1 |
| Mean ± SD | 60.35 ± 10.9 | 17M/3F | 165.7 ± 9.5 | 78.4 ± 8.9 | 28.81 ± 4.63 | 34.7 ± 48.00 | 26.75± 5.23 | - | - | - |
| Median | 60.5 | - | 165 | 79 | 27.8 | 24 | 28.5 | - | - | - |
| Percentage (%) | - | 85%M | - | - | - | - | - | 10% Used walking aid | 70% R | 35% MAS ≥ 2 |
Subjects’ Characteristics
3.2. Study Design
A one-group pre-test/post-test design was used in this clinical trial. The subjects were informed about all the procedures before testing. Subjects’ characteristics and relevant information were obtained prior to testing. To provide a detailed description of the sample characteristics, the following assessments were administered: plantar-flexor spasticity using Modified Ashworth Scale (MAS) and lower extremity function using Fugl-Meyer Assessment (FMA-LE).
3.3. Intervention
The assessment was performed using the TUG test by changing the foot placement, the subject was asked to sit on a standard chair, and the patient’s feet were placed on a white line marked on the floor. The patient sat on a standard chair, and when the test began, the patient stood up from the chair, walked a distance of 3 meters at the desired speed, and then sat down again. The duration of the TUG test was recorded by chronometer by changing the place of the foot at the beginning of the test. Starting position of the test was the following four models: 1- SP (Spontaneous foot position, the position in which the person voluntarily placed the legs), 2- SYP (Symmetrical foot position), 3- PBNP (Paretic foot behind the nonparetic one) and 4- NPBP (Nonparetic foot behind the paretic one). For the asymmetrical position, the base of the foot was placed about 50% behind the other foot (5). The sequence of foot positions in the TUG test was randomly selected, and each test was repeated three times. The subjects were given 5 - 10 minutes between tests to break (5). The tDCS device used in this study was an Activadose model and manufactured by Activa Tek in Taiwan. When tDCS was applied, direct current stimulation was transferred by a pair of electrodes (cathode and anode). The current was controlled by an ammeter. The stimulation electrodes were 5 x 7 cm and consisted of a sponge soaked in saline solution. While using tDCS, a flow of 1.5 mA was applied to the cerebellum for a maximum of 20 minutes (26), and the amount of electrical stimulation transmitted to the brain cells through the skull increased or decreased the excitability, which depended on the direction and intensity of electrical current (27). To prepare the skin and reduce the resistance of the skin, the hair of the desired area should be removed as much as possible, and the skin of the area should be cleaned and moist (28). When tDCS was applied to the cerebellum, the active anode electrode longitudinally was placed 2 cm blew the inion of occiput bone, and 1cm on the medial side of mastoid bone on the posterior of the head, which is exactly on the ipsi_lesional side and the other electrode was placed on the ipsilateral arm.
3.4. Statistical Analysis
Data analyses were performed using SPSS version 23 (IBM Corp., Armonk, N.Y., USA). Descriptive statistics were computed for demographic characteristics and for all variables (Table 1). Reliability analysis for all data was performed by intra-class correlations (ICC) in a group consisting of all subjects. All ICCs were above 0.80. We calculated the mean values of the time of the TUG test, over two trials. The paired t-test was used for comparing means of the continuous data of the time of TUG test before and after intervention in any foot positions separately. A P<0.05 was considered statistically significant.
4. Results
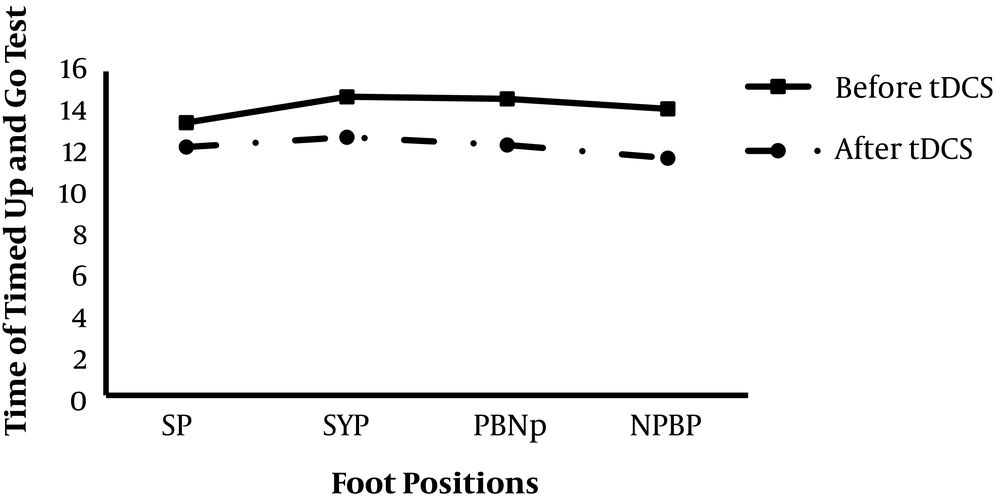
Twenty subjects aged 36 - 79 years (mean age = 60.35 ± 10.9 years) participated in the study. Most subjects were males (85%) who did not use a walking aid (90%). Also, 70% of the subjects were found with left hemisphere stroke. The baseline characteristics of the participants are detailed in Table 1. No sensitivity or an adverse reaction (rash, skin tear, or redness) was observed after using tDCS in the subjects. A Paired t-test was used to assess the baseline value of the time of the TUG test in each foot position (SP, SYP, PBNP, and NPBP), and its value significantly changed after the intervention (Table 2). This finding indicates that the time of TUG tests was decreased in all foot conditions after using one session of cerebellum tDCS (Figure 1).
| Different Foot Positions | Before Intervention | After Intervention | P-Value |
|---|---|---|---|
| SP | 13.47 ± 8.33 | 12.27 ± 7.45 | 0.012 |
| SYP | 14.75 ± 10.03 | 12.75 ± 8.60 | 0.010 |
| PBNP | 14.65 ± 11.11 | 12.32 ± 6.97 | 0.047 |
| NPBP | 14.15 ± 9.73 | 11.72 ± 6.79 | 0.037 |
Comparing Mean and SD of TUG Test Before and After Intervention with Different Foot Positions Using Paired t-Test
5. Discussion
Using tDCS on the cerebellum has been reported effective in reducing the time of the TUG test regardless of the foot position. In other words, tDCS is a useful method in improving functional balance. tDCS is a non-invasive technique, which modulates the excitability of the cerebral cortex (29). This method is safe and expensive, which can be used during exercise therapy and includes a low amplitude of electrical stimulation with the sponge electrodes soaked in saline solution. Electrical effects are moved from positive pole to negative pole and pass through the skull and reach the cerebellum (30). Although most of the currents are scattered along the path, among various tissues, an adequate amount of currents reaches the brain structures and change the membrane potential of the surrounding cells (30).
Studies have shown that different cortical regions, such as the premotor and supplementary motor cortex, the primary motor cortex (M1), cerebellum, and basal ganglia are part of a network, which plays a role in the acquisition of motor skills during motor learning (31, 32). Some studies have indicated that M1-atDCS improves motor performance and motor learning (33, 34).
Other studies also reported an enhancement in motor learning following cerebellar a-tDCS (35, 36). The cerebellum contributes to procedural motor learning and plays a critical role in structuring motor skills, perceptions, and motor behavior (35). The cerebellum plays a major role in decreasing errors related to new environmental demands during the motor learning process (37). M1 region partakes to the motor adaptation of skills by correcting errors during motor learning (18). Galea et al. (2011) compared the effects (online and short-term offline) of a-tDCS of cerebellum and M1 on motor learning during a visuomotor task. They found that cerebellar a-tDCS caused faster adaptation to the visuomotor task, while M1-a-tDCS enhanced retention of the newly learned visuomotor task (18).
Following a stroke, the symmetry of the sit-to-stand transition improves by placing the affected foot behind the unaffected foot (38). Generally, asymmetric vertical force is reported in the middle of performing a sit-to-stand transition. If the affected foot is placed behind, this asymmetric force decreases, and if the unaffected foot is placed behind, this asymmetric force increases. Thus, the foot placement during training should be considered (39). Recent studies have shown that using tDCS facilitates the upper limb movements in stroke subjects (40, 41). However, the effect of tDCS on lower limb function has been studied less than upper limb function. Other studies on balance, gait, and function in subjects with cerebral palsy and stroke in different models have also been done (14, 42-46). Dumont et al. reported a reduction in anteroposterior sways by applying tDCS on the primary motor cortex plus treadmill training in stroke subjects (42). Thus, applying a single session of cortical electrical stimulation was effective in static balance improvement. In Dumont et al. study, tDCS was applied on the primary motor cortex, while in the present study, it was used on the cerebellum. Similar to Dumont et al., Grecco et al. applied tDCS over the motor cortex of cerebral palsy subjects and reported an improvement in static balance and functional activities (14). In Grecco et al. study, there was a difference in placing the electrode and applying tDCS compared with treadmill training. Other studies have also been carried out on extensor strength and general stability in stroke patients, and after a single session of anodal electrical stimulation, there was an increase in knee extensors strength and general static stability (43, 47). All studies mentioned above have been effective in improving static balance in stroke patients and the function of cerebral palsy in children after a single session of anode tDCS. However, in these studies, electrical stimulation was applied on the primary motor cortex, and they were different from the present study, in which tDCS was used on the cerebellum. Studies also reported postural improvement in healthy cases due to tDCS (48). Short-term application of tDCS on the cerebellum caused standing balance improvement in stroke subjects (49); thus, it is consistent with this present study, in which using anodal tDCS on cerebellum in stroke patients improved functional balance.
5.1. Limitation
The results of this study cannot be generalized to acute and subacute patients, owing to the use of chronic subjects. In addition, ischemic and hemorrhagic patients in this study were not separated. Therefore, separation of patients and applying this intervention in different stages of stroke in these subjects can be helpful to provide appropriate treatment methods to improve the functional balance of this group of patients in the future. Also, there is a need for a control group to compare tDCS benefits. In addition, aging is an important factor in the balance; therefore, it should be considered in future studies.
5.2. Conclusion
The use of a single session of anodal tDCS on the cerebellum has been beneficial in patients with chronic stroke; therefore, it is recommended that physiotherapists improve functional balance in stroke subjects using tDCS.