1. Background
High blood pressure, or the chronic increase in arterial pressure at rest to above 140 mmHg for systolic and/or 90 mmHg for diastolic blood pressure, is one of the most notable and modifiable risk factors for cardiovascular disease (i.e., coronary artery disease, stroke, and heart failure) (1). The prevalence of high blood pressure varies by ethnicity and gender, but was reported between 25% and 43% in the US population (2) and 17.37% in Iran in 2006 (3). Patients with hypertension have significant changes in their cardiovascular systems in comparison with healthy people, which is mainly addressed by reduced vascular capacity and congested pulmonary arteries (3). This condition, if not treated appropriately, may lead to mortality in 50% of patients with hypertension from coronary artery disease or heart failure, 33% from stroke, and 10 - 15% from kidney failure (1, 2). Although hypertension is a chronic disease associated with many complications, using cost-effective strategies for controlling this disease seems necessary (4). Despite that antihypertensive drugs are effective and often have minimal side effects, health care costs are rising (1). Treatment guidelines for primary and secondary prevention of high blood pressure recommend that lifestyle changes, including increased physical activity, should be considered as the first-line non-pharmacological treatment. Considerable evidence indicates that regular exercise reduces systolic and diastolic blood pressure (5). The exercise-induced antihypertensive effects are often reported in people doing endurance exercises: a reduction of 5-7 mmHg after an exercise session (immediate effect) or after an exercise period (long-lasting effect) (6, 7).
The mechanisms proposed for exercise-induced antihypertensive effects include hormonal-neurological, vascular, and structural adaptations (8, 9). Reduced catecholamine and overall peripheral vascular resistance, improved insulin sensitivity, altered vasodilators, and vasoconstrictors, and decreased sympathetic nerve activity are some of the hypothesized explanations for exercise-induced antihypertensive effects (1, 2). Studies show that the amounts of moving mass and muscle strength are higher in eccentric contractions than in concentric contractions (10). Aerobic concentric exercises have been employed safely and successfully in patients with coronary artery disease for more than three decades. Eccentric exercises are also recommended as a new approach to increase muscle mass and muscle strength in patients with coronary artery disease (11). The research results show that the maximum cardiovascular and pulmonary responses, as well as systolic blood pressure and mean arterial pressure, were significantly lower during severe eccentric exercises than in concentric resistance exercises in the same workload in young adults (12) and diabetic patients (13). This feature makes eccentric aerobic exercises suitable for long-term rehabilitation (14). The angiogenesis function of eccentric exercises has been recently studied (15), while it was expected that with the expansion of the capillary network, the resistance in the vascular circuit is reduced. Considering the results of the studies, it seems that eccentric exercises are more appropriate and safer than concentric exercises to reduce blood pressure, and they can be administered in physiotherapy clinics to decrease orthopedic problems in patients with hypertension. To the best of the authors’ knowledge, no study has been conducted so far on the comparison between the effects of these two types of exercises in hypertensive individuals. The study hypotheses are as follows:
1- Concentric exercise can affect blood pressure in hypertensive patients.
2- Eccentric exercise can affect blood pressure in hypertensive patients.
3- Concentric and eccentric exercises have different effects on blood pressure in hypertensive patients.
2. Methods
2.1. Study Design
The current randomized, double-blind controlled clinical trial was conducted on three groups of patients matched by age, gender, and blood pressure. Both patients and observers were blinded to group allocation. This study was approved by the Ethics Committee of Semnan University of Medical Sciences (93/538814) and was registered in the Iranian Registry of Clinical Trials (IRCT number: 2014061618108N1).
2.2. Participants
The sample size was calculated according to a previous study by Madan Bhavna et al. (12) using the G*Power statistical package (version 3.0.10) with an α value of 0.05 to achieve a statistical power of 0.80 and an effect size of 0.80. The following options were also selected: test family, t test; statistical test, mean difference between two independent means; type of power analysis, a priori. The mean ± standard deviation of the mean heart rate in two hypertensive groups was 82.3 ± 11.66 pulses per second (pps) and 78.2 ± 8.48 pps, respectively (16). The results of the difference between the two groups revealed that a minimum of 15 participants was needed for each group. Therefore, to achieve an 80% power and a confidence level of 95% with an alpha level of P ≤ 0.05 as significant, 45 participants were examined in this study.
The Cardiovascular Department of Kowsar teaching hospital was the research setting of the current study. Before the study, the volunteers were familiarized with the objectives, conditions, and stages of the study, and they were asked to sign a form containing information about the project and the terms of voluntary participation. Then, they were randomly assigned to one of the three groups of eccentric exercise, concentric exercise, or control group as per the experimental protocol so that all the groups were matched by age, gender, and degree of disability.
The inclusion criteria were an age of 30-65 years, a body mass index (BMI) of 18.5 - 25, a mild to moderate chronic high blood pressure (over one year) with taking medication to control blood pressure, no history of regular exercise in the past six months (17), and no cardiovascular disease and diabetes. The participants were excluded if they had chest pain during exercises, any cardiovascular complications during the intervention, and any reason to discontinue the intervention. Volunteers were asked to refrain from consuming caffeine and energy drinks, and food two hours before exercise (18). They were also advised not to change their diets during the study, continue the same level of physical activity, and adhere to their regular lifestyles (19). All patients received medication (as commonly used drugs for angiotensin converting enzyme (ACE) inhibitors, beta-blockers, and diuretics) twice daily (in the morning and at night). The exercise sessions were performed four to six hours before and after receiving medication. To prevent differences caused by hour changes, the measurements were made at the same hour for all the groups (20). Participants were not aware of the group they were assigned to, and the observer (the person who evaluated outcome measures) was blinded to group assignment. Therefore, this was a double-blind study.
2.3. Intervention
First, the BMI was calculated for each subject. If it was in the desired range, a 24-h blood pressure device was placed on the wrist of the patient to examine the 24-h blood pressure to check if it is within the limits (20). After 24 h, the baseline blood pressure of each eligible participant was measured. For this purpose, the participant relaxed for 20 min while leaning back on a chair. Within the last 10 minutes of the rest time, the blood pressure was measured every two minutes by a digital arm blood pressure monitor (while the arm was at the heart level). The maximum and minimum blood pressure levels were deleted, and the last three blood pressure measures were reported. To increase the reliability of the results, the test was performed again on two different days (21). Then, the subjects walked on a treadmill for 30 min at their own speed starting from 1.5 km/h for early paces, which gradually increased to persuade the subjects to walk comfortably, based on their assigned group (18). After the familiarization session, the walking speed was chosen by the subject, starting from 1.5 km/h for the early paces, which gradually increased to 2.5 km/h, followed by increments every 30 s by 0.2 km/h until the subject announced that he could not tolerate the speed for more than 30 min. During the exercise, the subjects were encouraged to speed up walking until the heart rate raised by 60 - 85% of the MHR (22). The respiratory gases were collected within the last three minutes using a respiratory gas analyzer (AD instrument, ML206, Australia) (18). The test was performed in 12 min, and the workload was increased every three minutes. The VO2max (maximal oxygen consumption) for each person was considered when one of the following conditions fulfilled: Oxygen uptake reaching the Plato status, Respiratory Exchange Ratio (RER) exceeding 1:15, heart rate reaching its maximum (age -220), or maximum signs of dyspnea being met (20). This test was performed once after the first exercise session and once after the completion of the intervention, and then the values were compared.
After 30 min, the speed gradually decreased. In the current study, the immediate effect of exercise on blood pressure was studied. For this purpose, after an exercise session, the blood pressure of the individual, who was in the resting position for 20 min, was measured every two minutes in the last 10 min. At the end of the first session, a 24-h Holter blood pressure device was placed on the wrist of the patient to monitor the blood pressure changes within 24-h. Exercises were performed at 21°C (23). The subjects walked on the treadmill at speed chosen by themselves for four weeks, three 30-min sessions per week (18). Subjects were randomly assigned to one of the three following groups: +10% gradient (upward, for positive muscular function as the concentric group), -10% gradient (downward for negative muscular function as the eccentric group), and neutral gradient (as the control group). To evaluate the long-lasting effects of exercise on blood pressure, after the end of the four-week training course (12 sessions), the assessments were performed in a way similar to the first session. After 48 h, to evaluate the lasting effects of exercise, blood pressure was measured using a 24-h Holter blood pressure device for the third time. The mean heart rate was measured at all the stages. At all stages, systolic blood pressure (SBP) and diastolic blood pressure (DPB), as well as the Heart Rate (HR), were measured. After the first and the last exercise sessions, VO2max was also measured.
2.4. Statistical Analysis
Data were analyzed with SPSS version 19. The one-sample Shapiro-Wilk test was used to study the conformity of the frequency distribution of quantitative variables with normal theoretical distribution. Since all data were normal, the mean data of the study groups were analyzed using multivariate ANOVA, and the results were compared. The Tukey test was also used if there was an intragroup statistical difference (α ≤ 0.05). A 95% Confidence Interval (CI) was employed to compare the data between the study groups.
3. Results
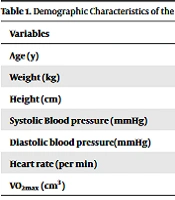
The results demonstrated that SBP had no significant difference between the three types of exercise (P = 0.759 and F = 278) (Table 1). However, regardless of the type of exercise, SBP significantly reduced in response to the increased activity during the four-week exercise period (P = 0.001) (Table 2).
| Variables | Neutral Gradient | Positive Gradient | Negative Gradient | F | P Value |
|---|---|---|---|---|---|
| Age (y) | 47.6 ± 7.85 | 45.86 ±7.08 | 47.4 ± 9.88 | 0.183 | 0.834 |
| Weight (kg) | 74.24 ± 8.99 | 79.94 ± 7.14 | 74.15 ± 9 | 2.172 | 0.127 |
| Height (cm) | 164.8 ± 7.94 | 166.9 ± 7.37 | 166.66 ± 6.93 | 0.344 | 0.711 |
| Systolic Blood pressure (mmHg) | 135.1 ± 6.22 | 136.9 ± 4.05 | 135.4 ± 6.03 | 0.474 | 0.626 |
| Diastolic blood pressure(mmHg) | 86.3 ± 2.86 | 86.6 ± 4.31 | 86.4 ± 2.85 | 0.032 | 0.969 |
| Heart rate (per min) | 73.87 ± 6.71 | 74.80 ±7.49 | 73.80 ± 6.39 | 0.099 | 0.906 |
| VO2max (cm3) | 29.99 ± 4.57 | 29.98 ± 4.23 | 29.84 ±4.07 | 0.017 | 0.993 |
| Variable | (I) group | (J) group | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| MDBP in the follow-up | Neutral | Positive | -2.72778 a | 0.95678 | 0.020 | -5.1137 | -0.3419 |
| Negative | 0.59167 | 0.95678 | 1.000 | -1.7942 | 2.9776 | ||
| Positive | Neutral | 2.72778 a | 0.95678 | 0.020 | 0.3419 | 5.1137 | |
| Negative | 3.31944 a | 0.95678 | 0.004 | 0.9336 | 5.7053 | ||
| Negative | Neutral | -0.59167 | 0.95678 | 1.000 | -2.9776 | 1.7942 | |
| Positive | -3.31944 a | 0.95678 | 0.004 | -5.7053 | -0.9336 | ||
Abbreviation: MDBP, Mean Diastolic Blood Pressure.
aP value is significant at the 0.05 level.
Diastolic blood pressure showed no significant difference between the three types of exercise in the pre-intervention stage, after the first session of intervention, and at the end of the intervention (Table 3). However, in the follow-up, DBP was different between the groups (P = 003). The results demonstrated that DBP was different between neutral and positive gradient groups (P = 0.02), and between positive and negative gradient groups (P = 0.004) (Table 2). Besides, DBP was significantly higher in the positive gradient group than in the negative and neutral gradient groups in the follow-up stage. However, regardless of the type of exercise, DBP decreased significantly in response to increased activity during the four weeks (P = 0.001). Moreover, HR had no significant difference between the three types of exercise (P = 0.873, F = 136.2). However, regardless of the type of exercise, HR increased significantly in response to physical activity (P = 0.001). Besides, VO2max had no significant difference between the three types of exercise (P = 0.539, F = 0.627). Neutral and positive gradient exercises significantly increased VO2max (P < 0.05) (Table 4).
| Mean | Std. Deviation | 95% Confidence Interval for Mean | Between Groups | |||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | F | P Value | |||
| MSBP pre | 0.47 | 0.6 | ||||
| Neutral | 135.13 | 6.22 | 131.68 | 138.57 | ||
| Positive | 136.95 | 4.05 | 134.70 | 139.19 | ||
| Negative | 135.40 | 6.03 | 132.06 | 138.75 | ||
| MDBP pre | 0.03 | 0.9 | ||||
| Neutral | 86.28 | 2.85 | 84.7 | 87.8 | ||
| Positive | 86.58 | 4.30 | 84.2 | 88.9 | ||
| Negative | 86.35 | 2.85 | 84.7 | 87.9 | ||
| MSBP after the first session | 1.3 | 0.2 | ||||
| Neutral | 130.12 | 6.02 | 126.7 | 133.45 | ||
| Positive | 133.18 | 5.94 | 129.8 | 136.47 | ||
| Negative | 129.92 | 6.15 | 126.51 | 133.32 | ||
| MDBP after the first session | 1.4 | 0.2 | ||||
| Neutral | 81.78 | 3.23 | 79.99 | 83.57 | ||
| Positive | 84.13 | 6.01 | 80.80 | 87.46 | ||
| Negative | 81.73 | 3.35 | 79.87 | 83.59 | ||
| MSBP after the last session | 1.5 | 0.2 | ||||
| Neutral | 126.5 | 4.88 | 123.81 | 129.22 | ||
| Positive | 129.8 | 7.75 | 125.50 | 134.09 | ||
| Negative | 126.45 | 4.89 | 123.74 | 129.16 | ||
| MDBP after the last session | 0.07 | 0.9 | ||||
| Neutral | 78.5 | 2.67 | 77.02 | 79.98 | ||
| Positive | 78.98 | 6.84 | 75.19 | 82.77 | ||
| Negative | 78.41 | 2.62 | 76.95 | 79.86 | ||
| MSBP in the follow-up | 2.5 | 0.09 | ||||
| Neutral | 132.4 | 2.74 | 130.88 | 133.92 | ||
| Positive | 134.51 | 4.47 | 132.03 | 136.99 | ||
| Negative | 131.89 | 2.52 | 130.5 | 133.29 | ||
| MDBP in the follow-up | 6.8 | 0.003 | ||||
| Neutral | 80.9 | 2.00 | 79.81 | 82.02 | ||
| Positive | 83.64 | 3.73 | 81.5 | 85.71 | ||
| Negative | 80.32 | 1.61 | 79.43 | 81.22 | ||
Abbreviations: MSBP, mean systolic blood pressure; MDBP, mean diastolic blood pressure.
aSignificant at P < 0.05
| Mean | Std. Deviation | 95% Confidence Interval for Mean | Between Groups | |||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | F | P Value | |||
| AHR in pre- intervention | 0.014 | 0.9 | ||||
| Neutral | 131.66 | 8.59 | 126.90 | 136.42 | ||
| Positive | 132.06 | 10.14 | 126.44 | 137.68 | ||
| Negative | 131.53 | 8.26 | 126.95 | 136.10 | ||
| AHR after the first session | 0.370 | 0.6 | ||||
| Neutral | 73.4 | 5.80 | 70.18 | 76.61 | ||
| Positive | 74.4 | 6.53 | 70.78 | 78.01 | ||
| Negative | 72.6 | 4.74 | 69.97 | 75.22 | ||
| AHR after the last session | 0.016 | 0.9 | ||||
| Neutral | 132.8 | 8.14 | 128.35 | 137.37 | ||
| Positive | 132.4 | 10.35 | 126.73 | 138.2 | ||
| Negative | 133.4 | 7.14 | 129.44 | 137.35 | ||
| AHR 48 h after the last session | 0.044 | 0.9 | ||||
| Neutral | 73.86 | 6.70 | 70.15 | 77.58 | ||
| Positive | 74.8 | 7.49 | 70.64 | 78.95 | ||
| Negative | 73.8 | 6.39 | 70.25 | 77.34 | ||
| VO2max after the first session | 0.099 | 0.9 | ||||
| Neutral | 30.6 | 5.08 | 27.78 | 33.41 | ||
| Positive | 30.76 | 4.83 | 28.08 | 33.44 | ||
| Negative | 30.75 | 3.93 | 28.71 | 33.08 | ||
| VO2max after the last session | 0.007 | 0.9 | ||||
| Neutral | 30.02 | 4.54 | 27.50 | 32.53 | ||
| Positive | 29.98 | 4.23 | 27.64 | 32.33 | ||
| Negative | 29.84 | 4.06 | 27.59 | 32.10 | ||
Abbreviation: AHR; Average Heart Rate.
aSignificant at P < 0.05.
4. Discussion
Similar to previous studies, the present study showed that all three types of exercise significantly affected SBP, DBP, and HR indices immediately and 48 h after the intervention. However, the findings of the current study showed no significant differences between the study groups regarding the effects of the three types of exercise on SBP, DBP, HR, and VO2max measures, except for DBP in the 48-h follow-up after the last session.
First, the results of this study showed that systolic and diastolic blood pressures, average heart rate, and VO2max decreased after four weeks of aerobic training, except for VO2max in the neutral gradient group. Previous studies showed that one session of eccentric exercise could decrease blood pressure, mean systolic and diastolic arterial pressure, and heart rate (6-8, 10, 12, 13, 18, 24). However, our results are contrary to the studies by Okamoto et al. (6, 7, 10), Gault et al. (18), and Bhavna et al. (12). The differences in the results can be explained by the exercise protocols, duration of exercise training, and participants’ conditions such as disease and age. For example, Bhavna et al. evaluated the effects of concentric and eccentric exercises on four muscle groups: included biceps, deltoid, quadriceps, hip abductors through the weight cuff, dumbbells and quadriceps table (three sets of 10 repetitions with one-minute rest between each set) at 75% of the 10-repetition maximum. However, we employed a different exercise regimen including treadmill walking (an aerobic exercise). Muscle-strengthening exercise with a dumbbell might result in fewer cardiovascular responses; therefore, different results might be inevitable.
Another result of this study was that no difference was observed in SBP, DBP, average heart rate, and VO2max between the groups pre-intervention, after session 1, after the last session, and in the follow-up, except for DBP. Therefore, the results demonstrated that DBP was higher in the follow-up in the positive gradient group than in the negative and neutral gradient groups. These results are contrary to the study by Katharina et al. (8) and Gault et al. (18). Gault et al. compared neutral and -10% gradient treadmill walking at the desired speed for 15 min. They showed that the oxygen intake rate was 25% lower during downward walking than in 0% gradient walking. In addition, lower amounts of stroke volume, cardiac output, arteriovenous oxygen difference, and SBP were reported at the -10% gradient. However, no difference was reported in HR, DBP, and mean arterial pressure between the groups (18). They stated that downward walking had lower cardiac strain (cardiac output, stroke volume, SBP), endothelial changes, and metabolic demand than walking on flat surfaces. Therefore, the reduction in the BP levels was due to the reduction in peripheral vascular resistance or cardiac output (25). Contrary to their study, our study demonstrated different DBP between the groups after the intervention. They examined cardio-respiratory responses after one session of walking exercise. In our study, cardio-respiratory responses were evaluated after four weeks of walking exercise. Similarly, Okamoto et al. (2008, 2009) (6, 19), comparing the effect of resistance exercises, concluded that eccentric resistance exercise had a greater impact on decreasing arterial stiffness and blood pressure than concentric resistance exercise. This can be because arterial stiffening might be a possible consequence of concentric aerobic exercise training in four weeks (9). In another study, Blazevich et al. (2017) showed that eccentric cycling for one session needed less oxygen intake and caused fewer changes in the heart rate than concentric cycling (26). Some differences might be explained by different protocols, including the number of exercise sessions and the type of aerobic training. However, Coelho et al. (2017) showed no differences between aerobic and resistance training in hemodynamic and cardio-respiratory evaluations (27).
4.1. Limitations
Our study has some potential strengths and limitations. Our study assessed the long-lasting effects of aerobic training on BP and other cardiac variables. However, we did not have a follow-up longer than 48 h after the exercise protocol.