1. Context
Lichen planus (LP), as a relatively common chronic inflammatory disease, can probably indicate a cell-mediated immune response to antigenic changes induced in the skin or mucus of susceptible individuals (1). This disease was firstly described in 1869, and its exact mechanism is not well understood yet (2). Although skin lesions are self-limited in most cases with this disease, oral lesions whose control is more difficult are chronic and often resistant to conventional therapies, morbidities (3). WHO classified oral lichen planus (OLP) as a potentially pre-malignant disease (1). Moreover, OLP is clinically seen in two non-erosive and erosive forms (4). In OLP, large amounts of cytokines are secreted that play key roles in the development, continuity, exacerbation, and characterization of the disease-related conditions (5). The role of the imbalance of these secreted cytokines in the pathogenesis of disease has been hypothesized in the late 1980s. Accordingly, as this imbalance can lead to disease, cytokine modification can be considered as a suitable and important therapeutic strategy for these diseases (6).
The changes occur at the molecular level after genetic variations, which are before morphological and histopathological changes. Therefore, measuring and analyzing cytokines at different levels, including DNA, mRNA, and protein, can play positive roles in early diagnosing the disease, monitoring the disease’s activity, and determining the response to treatment (7). In this regard, one of the key cytokines is tumor necrosis factor-alpha (TNF-α), which is a major mediator in acute inflammation and antitumor immunity (8). This cytokine has two receptors of TNF R-2 and TNF R-1. Notably, the synergistic effect of these two receptors is required for cell death (9). TNF-α is a multifunctional cytokine playing significant roles in immunity, host defense responses to infection (10, 11), inflammation, apoptosis, the development and balance of some organs, stimulation of angiogenesis, and affecting tissue remodeling and adjustment of cell proliferation and differentiation (12).
The TNF-α was found to be effective on the pathogenesis of many inflammatory, pre-cancerous, cancerous, and autoimmune diseases (13). Studies on the relationship between OLP and TNF-α have reported many problems such as uncertainties in the definitive diagnosis of OLP, the separate study of TNF-α levels in courses and different types of OLP, and the lack of simultaneously examining TNF-α levels and the related receptors. Additionally, the changes in levels of cytokines are used to determine the efficacy of treatments. To the best of our knowledge, no review articles have been done on evaluating TNF-α levels before and after performing standard treatments of OLP. Therefore, this study aimed to provide for the first time a relatively complete review on the association of this cytokine with OLP etiopathogenesis, by focusing on the types of TNF-α-secreting cells, the types of medium, and levels. Moreover, this was done to evaluate the TNF-α and determine the role of therapeutic agents in improving the OLP lesions by measuring the level of this cytokine.
2. Evidence Acquisition
Some databases, including PubMed, Google Scholar, Scopus, and Embase (Ovid) with keywords of oral lichen planus, NF- κB, OLP, TNF-α, and Tumor Necrosis Factor-alpha without time limit (2017 - 1900) were searched to collect the required data from the related articles.
The inclusion criteria were original article; confirmation of OLP diagnosed based on the modified WHO or krunch-koff and Eisenberg criteria; measurement of TNF-α levels in at least one medium such as saliva, serum, transudate or tissue specimens; evaluation of TNF-α levels before and after the application of a therapeutic method; and TNF-α levels in one of the levels of DNA, mRNA, and protein. Besides, two reviewers evaluated each article included in this study and then judged them to determine the risk of bias of the article based on the following three items: (1) Features of the study; (2) Consistency of the control group; (3) Prospective design.
Three categories of met (included in the study), unmet (excluded from the study), and unclear (not referred to in the study) were also considered for scoring each one of these items. The studies were categorized into the following three groups after being validated: (1) Low risk of bias: all items are met; (2) Moderate risk of bias: one or two items are unclear; (3) High risk of bias: at least one item is not met or all three items are unclear.
These assessments and validations of the articles were performed without blinding the author name, the institution, and the journal name. The results are shown in Table 1.
| Reference (First Author and Year) | Study Group | Control Group | Study Design | Risk of Bias |
|---|---|---|---|---|
| Ghallab 2010 (9) | Unclear | Met | Met | Moderate |
| Rhodus 2006 (14) | Unclear | Unclear | Met | Moderate |
| AL-MOHAYA 2015 (15) | Unclear | Unclear | Met | Moderate |
| Pezelj-Ribaric 2004 (12) | Unclear | Unclear | Met | Moderate |
| Sklavounou 2000 (16) | Unclear | Unmet | Met | Low |
| Cao 2016 (17) | Unclear | Unclear | Met | Moderate |
| Pekiner 2012 (5) | Unclear | Met | Met | Moderate |
| Yamamoto 1994 (18) | Unclear | Unclear | Met | Moderate |
| Kalogerakou 2008 (19) | Unclear | Unclear | Met | Moderate |
| Thongprasom 2006 (11) | Unclear | Unclear | Met | Moderate |
| Yamamoto 1995 (20) | Unclear | Unclear | Met | Moderate |
| Amer 2009 (21) | Unclear | Unclear | Met | Moderate |
| Aghahosseini 2011 (10) | Met | Met | Met | High |
| Othman 2016 (22) | Unclear | Unclear | Met | Moderate |
3. Result
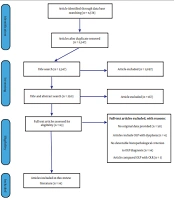
Of 350 abstracts, 82 articles related to the subject of this study were identified and their full texts were extracted in PDF format. Thereafter, 70 articles were excluded due to not meeting the inclusion criteria. Finally, 14 completely related articles that met the inclusion criteria, were included in this study (Figure 1, Tables 2 and 3).
| Country | Author & Year | Case | Control | Provenance of Control | Sex, Case Group | Sex, Control Group | Age in Case Group | Age in Control Group | Clinical Type of OLP | OLP Criterion | Medium | Level | Test | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egypt (9) | Ghallab 2010 | 20 | 20 | Unknown | M/F, 2/18 | M/F, 3/17 | 40 - 5546.3 ± 4.99 | 39 - 5542 ± 7.2 | Erosive | WHO | Saliva | Protein | ELISA | TNF-α &TNFR-2 OLP > control, significantly; decreased significantly posttreatment |
| USA (14) | Rhodus 2006 | 13 | 13 | Unknown | F | F | 57.2 years (range: 28 - 78) | Erosive-ulcerative | KrutchkoffEisenberg | Saliva | Protein | ELISA | OLP > control, significantly; decreased significantly posttreatment | |
| Saudi Arabia (15) | AL-MOHAYA 2015 | 42 | 211 | Blood donor | M/F, 16/26 | M/F, 140/71 | 27 - 72 | 20 - 65 | Reticular, erosive, atropic, bullos plaque | WHO | Blood | DNA | (ARMS)-PCR | (-308G/A) SNP are associated with OLP risk. |
| Croatia (12) | Pezelj-Ribaric 2004 | 40 | 20 | Unknown | - | - | - | - | Reticular, erosiveatrophic | WHO | Serum | Protein | ELISA | OLP > control; erosive/atrophic > reticular, significantly |
| Greece (16) | Sklavounou 2000 | 22 | 10 | Fibrous hyperplasias | M/F 7/15 | - | 41- 75 (mean age 56.9) | - | Reticular | WHO | Tissue biopsy | Protein | IHC | OLP > control, significantly |
| China (17) | Cao 2016 | 48 | Wisdom teeth extraction | M/F 20/28 | <40 (19–39) ≥40 (40–71) | 18 to 35 | Reticular | WHO | Tissue biopsy | Protein | ELISA | OLP keratinocytes > control keratinocytes, significantly | ||
| Turkey (5) | Pekiner 2012 | 30 | 30 | Unknown | M/F 9/21 | M/F 12/18 | 51.10 ± 12.25 | 48.09 ± 11.92 | Reticular, erosive, bullous | WHO | Serum | Protein | ELISA | OLP > control, no-significantly |
| Japan (18) | Yamamoto 1994 | OLP:26, SCC:30 | 20 | Unknown | M/F, 6/20, 21/9 | M/F, 10/10 | 25 - 82, 40-89 | 25 - 48 | - | WHO | Blood | Protein | ELISA | OLP & SCC > control, significantly; OLP similar SCC |
| Greece (19) | Kalogerakou 2008 | 80 | 80 | Healthy volunteers | M/F, 49/31 | M/F, 30/30 | 55.6 ± 13.3, 18-76 | 50 ± 6.1, 20-65 | Reticular, plaque, atrophic,erosive | WHO | Blood | Protein | ELISA | Number of TNF-a secreting cells OLP > control; erosive > reticular; No-significantly |
| Thongprasom 2006 | 18 | 20 | M/F, 4/14 | 17 - 67 | Atrophic, erosive | WHO | Tissue biopsy | Protein | IHC | Number of mononuclear cells OLP>control, significantly; decreased significantly posttreatment | ||||
| Japan (20) | Yamamoto 1995 | 7 | 6; 6 | Inflamegingiva, Normal gingiva | WHO | Tissue biopsy | Protein | ELISA | OLP > control, significantly | |||||
| India (21) | Amer 2009 | 30 | 10 | Reticular, erosive | WHO | Serum/saliva | Protein | ELISA | OLP > control, significantly | |||||
| Iran (10) | Aghahosseini 2011 | 30 | - | - | M/F, 9/21 | - | 35 - 66 | - | Reticular, erosive | WHO | Saliva | Protein | ELISA | Decreased, no-significantly posttreatment |
| Egypt (22) | Othman 2016 | 12: laser, 12: corticosteroid | - | - | M/F, 6/18 | 35-70 | Reticular, plaque, atrophic, erosive | WHO | Serum | Protein | ELISA | Decreased significantly posttreatment in corticosteroid group | ||
| Excluded Studies | Author/ Year | Reason of Exclusion |
|---|---|---|
| 1 | Zhang 2008 (23) | Include OLP with dysplasia. |
| 2 | Carrozzo 2004 (24) | No detectable histopathological criterion to OLP diagnosis. |
| 3 | Rhodus 2005 (25) | Include OLP with dysplasia. |
| 4 | Simark-Mattsso 2012 (26) | No detectable histopathological criterion to OLP diagnosis. |
| 5 | Yanni Wang 2016 (27) | No detectable histopathological criterion to OLP diagnosis. |
| 6 | Ghalayani 2013 (28) | Compared OLP with OLR no control group. |
| 7 | Xavier 2007 (29) | No detectable histopathological criterion to OLP diagnosis. |
| 8 | Zhou 2009 (30) | No detectable histopathological criterion to OLP diagnosis. |
| 9 | Khan 2003 (31) | No detectable histopathological criterion to OLP diagnosis. |
| 10 | Kaur 2015 (32) | Include OLP with dysplasia. They applied WHO criteria for 1987 not 2003. |
| 11 | Malarkodi 2015 (13) | No detectable histopathological criterion to OLP diagnosis. |
| 12 | Rhodus 2005 (33) | Include OLP with dysplasia. |
| 13 | Chen 2007 (34) | No detectable histopathological criterion to OLP diagnosis. |
| 14 | Zhao 2001 (35) | No detectable histopathological criterion to OLP diagnosis. |
| 15 | Sun 2007 (36) | No detectable if they biopsy or no and histopathological criterion to OLP diagnosis. |
For performing meta-analysis on the data, Revman 5.3 software was used. The obtained data were entered as TNF-α level (pg/mL) and in the form of Mean ± SD. If I2 > 50% is reported, high heterogeneity of articles would be indicated, so random effect will be used, otherwise Fixed effect will be used to analyze the data. According to the meta-analysis performed by Mozaffari et al. in 2017 on the Salivary and serum levels of TNF-α in patients with oral lichen planus (37), in the present study, those articles that evaluated the effect of treatment on TNF-α levels in OLP patients were entered into the meta-analysis. Therefore, they reported cytokine levels before and after the treatment.
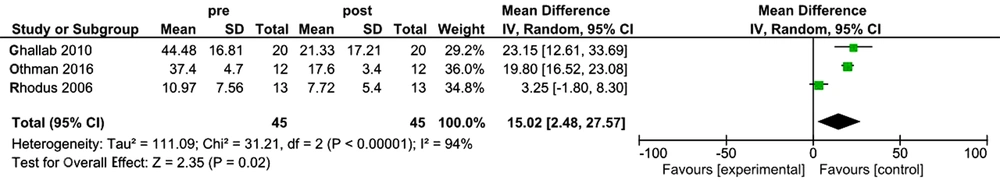
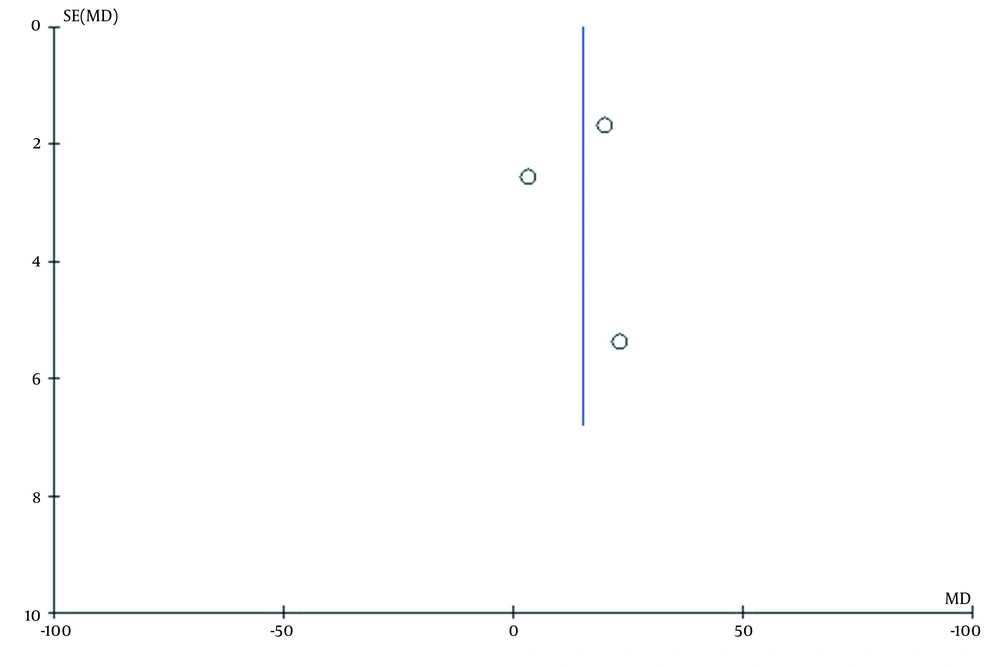
Out of 4 articles selected in this study, three studies that assessed the effect of the standard treatment of oral lichen planus were included in the meta-analysis. Since I2 = 94% is indicative of high levels of heterozygosity of articles, random effect test was used to analyze the data. The sample size was calculated as 45 patients with oral lichen planus. Mean difference and CL 95% were 15.02(2.48, 27.57), respectively. According to the analysis, P-value = 0.02 was found, indicating a significant effect of the standard treatment on TNF-α levels in OLP patients (Figure 2). As well, there was a publication bias due to the imbalance of studies on the graph of the Funnel Plot in the analyzed studies (Figure 3).
4. Discussion
The OLP is a T-cell mediated chronic inflammatory tissue response characterized by two specific and non-specific antigenic expression mechanisms. Correspondingly, many possible contributors resulting in these antigenic changes have been introduced so far (24).
In the current review article, the results indicate that the TNF-α levels in the OLP are significantly increased, suggesting that the increased level of this cytokine may be effective on initiating the disease and activating autoantibody-inducing mechanisms. Our result show that polymorphism in TNF-α gene can be considered as one of the effective factors leading to an increase in TNF-α levels. Since changes at the genetic level occur before morphological and functional changes, they can essentially be regarded as the most basic changes in TNF-α molecule. Hence, it seems that the use of therapeutic agents that can inhibit TNF-α expression at the genome level or prevent the function of the relevant transcription factors, can lead to OLP inhibition in basic levels.
TNF-α plays critical roles at various stages of etiopathogenesis of OLP.
- The expression of heat shock protein (HSP) as autoantigen on the keratinocyte surface, essentially reflects the TNF-α activity, since some genes like the HSP-70 are located in the TNF-α gene region (8).
- The specific Antigen (Ag) mechanism involves the release of cytokines by T-cells (CD4+, CD8+) that results in a cytotoxic reaction against the basal cell layer of the epidermis (36, 38).
- TNF-α leads to the increased expression of MHC-I and MHC-II on the surface of many cells (13).
- Differentiation of TCD8+ into cytotoxic T-cell (31, 39).
- The TNF-α binding to TNF-R-1 activates Nuclear Factor Kappa B (NF-Κb) (nuclear factor kappa-B) releasing into the nucleus and adhered on the promoter region of the anti-inflammatory genes (30, 40).
- High levels of TNF-α production lead to the increased S-Fas secretion, which inhibits the Fas/Fas-L system and ultimately prevents over-apoptosis of keratinocytes and T-cells (41). So, NF-κB leads to either chronicity or the increased severity of the OLP due to anti-apoptotic function in T-cells and localization of lesions by apoptosis inhibition in the keratinocytes. As a result, inflammation is much more likely to occur than necrosis in the OLP (17, 40, 42).
- The TNF-α derived from keratinocytes stimulates T-cell apoptosis by binding to TNF-R-1 and then by producing apoptotic signals. The disease’s activity is determined according to the balance between the keratinocyte apoptosis by T-cells-derived TNF-α and the T-cell apoptosis by keratinocytes-derived TNF-α in the epithelium (17, 31).
- TNF-α has significant effects on proliferation, differentiation, and maturation of B-cells and the associated immune responses (43, 44).
In addition to specific mechanisms, some non-specific mechanisms were also found to be effective on the OLP pathogenesis (36, 38). In this regard, the non-specific mechanism appears to begin with the degranulation of mast cells and the release of proinflammatory mediators. Moreover, TNF-α secreted by mast cells increases the production of matrix metallopeptidase (MMP) and MMP-like collagenases involved in the destruction of basement membranes increased production of MMP and MMP-like collagenases (39). Additionally, it is effective on leukocyte migration and inflammatory infiltration in and around the basement membrane by increasing the production of adhesion molecules such as E-selection and intercellular adhesion molecules (ICAM) (45). It was shown that TNF-α stimulates the secretion of (Related to the activation of normal T-cell expressed and secreted) RANTES and their receptors. Of note, RANTES binding to the receptor plays important roles in the migration and degranulation of mast cells as well as the secretion of TNF-α. This positive cycle plays a significant role in the chronicity of OLP. Furthermore, RANTES plays an important role in the recruitment of lymphocytes, monocytes, NK-Cells, eosinophils, basophils, and mast cells Increase RANTES (45). As well, it was indicated that NF-KB-dependent cytokines like TNF-α play important roles in up-regulating vascular endothelial growth factor (VEGF) expression, proliferation, migration, and degradation of endothelial cells and vascular permeability, which is crucial in initiating angiogenesis. Neo-angiogenesis is a common occurrence in chronic inflammatory diseases like OLP (8). Additionally, TNF-α produced by vascular wall and perivascular adipose tissue by increasing NADPH oxidase activity leads to an increase in O2 free radicals, thereby reducing nitric oxide (NO) production and increasing NO fracture, which ultimately severe inflammatory reactions (38).
Moreover, the non-specific mechanism-dependent reactions ultimately involve the induction, activation, proliferation, proliferation, and differentiation of T cells and their infiltration into the superficial lamina propria, destruction of the basement membrane, and the entry of immune cells into the epidermis and keratinocyte apoptosis (36, 38).
In the current review, 14 articles were evaluated based on the inclusion criteria (Table 1). Of them, only two studies compared the level of TNF-α producing cells in the case and control groups (11), and the others have examined the TNF-α levels (Table 1). In all these articles, the TNF-α levels or the count of TNF-α producing cells had increased in patients with OLP compared to the controls. Of note, these differences were not statistically significant only in two studies (5). The TNF-α producing cells are Th1, cytotoxic T-cells, mast cells, macrophages, monocytes (16, 36), B-cells, dendritic cells (CDs), natural killer cells, fibroblasts (36), endothelial cells (19, 36, 46), and basophils (8, 26).
According to the results of the articles collected in this study, the TNF-α has been studied at various levels of DNA, mRNA (polymorphism), and protein. Some articles reported that the polymorphism in -308 A/A had the highest levels of TNF-α (29, 47). In the present study, among the available articles that examined TNF-α at the levels of both DNA and mRNA, only the results of one study were evaluated according to the inclusion criteria (15). Other articles included in our study have examined TNF-α at the protein level (Table 2). It can be concluded that the TNF-α changes in the patients with OLP occur at both the DNA level (gene polymorphism) and the translation level (protein).
The articles studied in this review evaluated TNF-α in either one of the following media:
(1) Saliva: Increasing cytokines in saliva can be due to (1) local production of inflammatory cells to the lesion, (2) production by keratinocytes, and (3) loss of structural barriers in the oral mucosa (23). Three previous studies conducted in 2006, 2010, and 2011 used saliva samples to measure the level of TNF-α. Amer et al. in a research in 2009 studied the level of this cytokine using both blood and saliva samples (9, 10, 14, 21).
(2) Serum: the increased serum TNF-α levels can be due to the production of TNF-α from endothelial cells, monocytes, and circulating T-cells. As well, the leakage of this cytokine from the interstitial fluid to the serum via endothelial cell-to-cell junctions increases the serum TNF-α level (8). A number of authors in their studies also compared TNF-α levels between the years 1994 and 2015 in serum or blood samples (12, 15, 18, 19, 21, 22, 45).
(3) Transudate: None of the articles in this study used this medium (6).
(4) Biopsy: Yamamoto, Sklavounou, Cao, and Thongpasom measured the TNF-α levels in the biopsy samples obtained from the included patients and controls in their study using either ELISA assay or IHC method (11, 16, 17, 20).
Considering the similarity of the results related to the measurement of the TNF-α level in this review study (significant and/or non-significant increase in TNF-α levels in the OLP group compared to the control group), it can be stated that there is a statistically positive correlation among TNF-α levels in different media. Therefore, it can be concluded that the saliva collection is more non-invasive and cost-effective compared to other methods.
In addition to the articles that directly examined the TNF-α levels in patients with OLP, another category of articles evaluated the TNF-α level after applying therapeutic agents and then compared it with pre-treatment values, in order to confirm the role of this cytokine in the OLP pathogenesis. Considering that OLP is considered as a T-cell mediated autoimmune disease, so the use of therapeutic methods to modulate or suppress the immune system and inflammatory responses can be helpful, possibly reasonable, and sufficient. Some previous studies have used the measurement of TNF-α levels to monitor therapeutic response in OLP. As mentioned earlier, the TNF-α has a dual function in both the development and homeostasis of the body as well as a function in the inflammation, apoptosis, and destruction of the tissue. Among the articles that met the inclusion criteria of this study, the studies by Ghallab et al. (2010), Rhodus et al. (2006), and Thongprasom et al. (2006) demonstrated that TNF-α levels significantly decreased after treatment (9, 11, 14), confirming the importance of the TNF-α role in the OLP pathogenesis. Therefore, such studies might be exploited to determine both the efficacy of treatment and the prognosis of the disease.
When using anti-TNF-α medications, it should be considered that the complex interdependent relationship between cytokines can lead to paradoxical complications. For example, the TNF-α inhibition was found to cause immune imbalance and upregulation of other precursor cytokines like interferon-alpha (INF-α) that can result in the symptoms similar to OLP and other side effects. Based on the reported results of meta-analysis in this research, it can be said that evaluation of TNF-α level before and after of treatment is a good predictor to determine responsiveness to the performed treatment.
4.1. Limitations in the Current Research on TNF-α
The results of the studies reviewed in this study indicated an increase in the TNF-α level in the OLP patients and a reduction after performing the treatment, and no significant contradictory results were found. It should be noted that there were some limitations in most of these articles, which may affect the results, so they are recommended to be included in comprehensive studies in the future.
(1) The items affecting the levels of TNF-α are numerous, including the consumption of cinnamon, yogurt, alcohol, and cigarettes as well as recent illnesses, so as far as possible, these should be eliminated at sampling time or be identical between the case and control groups (13).
(2) The changes in the TNF-α levels at all levels (DNA, mRNA, and protein) may not coincide at the same time. In this study, none of the articles reviewed TNF-α simultaneously at several levels.
(3) Due to the controversial biological effects of TNF-α in the OLP etiopathogenesis on controlling the disease’s severity and limiting the lesions, future studies are needed by focusing on examining and comparing the level of this cytokine at different stages of the disease (41).
4.2. Some of the Benefits of Measuring the TNF-α Levels in Patients with OLP Are as Follows
- Prognosis of OLP malignancy. This cytokine plays roles in the angiogenesis and increased expression of VEGF, which is necessary for cell growth and carcinogenesis of OLP (8, 48, 49). Therefore, it is suggested to conduct further research on TNF-α as a predictive marker for the malignant transformation of OLP.
- Evaluation of the effectiveness of different treatments on OLP by measuring the TNF-α levels (9-11, 14):
- It can be said that one of the causes of simultaneous skin and oral mucus involvement may be the increased levels of TNF-α production. The patients with OLP with 308 TNF-α G/A genotype (82%) have higher skin involvement compared to the patients without polymorphisms (30%) 15.
- Investigating the risk of clinical signs in OLP patients. Rhodos (2006) reported that higher levels of TNF-α may be associated with pain and hyperalgesia in OLP patients (14).
4.3. Conclusions
Given that the standard treatments of OLP disease have reduced the TNF-α levels, which were higher in the OLP patients compared to normal subjects in all of the studies presented in this article, it can be suggested that the TNF-α levels may be useful in determining both prognosis and effectiveness of the treatment performed.