1. Background
Dementia is a disorder associated with a progressive decline in cognition, affecting daily functioning. Patients with different types of dementia may experience dysphagia over the course of the disease (1). Previous studies have reported that progressive cognitive deterioration and dysphagia may coincide at all stages of dementia. It has been shown that patients with mild to moderate dementia have inappropriate eating patterns because of failure to successfully manage either the food bolus in the mouth or aspiration upon swallowing (2). The disease’s severity is associated with a higher risk of aspiration pneumonia, leading to the patient’s death (3). Likewise, Rösler et al. evaluated the effects of dementia severity and food texture on the incidence and type of dysphagia in hospitalized elderly, reporting dysphagia as an important and common symptom in people with moderate to severe dementia (4). However, detailed information about the role of cognitive function in the occurrence of dysphagia in patients with dementia is still limited.
The role of cognition in dysphagia development after stroke has been described (5). In this regard, Ebrahimian et al. found that cognitive deficits played a role in dysphagia occurrence after stroke. Considering the varying nature of swallowing problems in patients with cognitive disorders and stroke-induced dysphagia (6), the symptoms of dysphagia may encompass irregular meal times to progressive weight loss. On the other hand, in primary dysphagia, symptoms include longer feeding periods, multiple swallows, smaller bites, and prolonged chewing (7). Therefore, it is valuable to identify the role of cognitive function in the occurrence of dysphagia in patients with cognitive disorders.
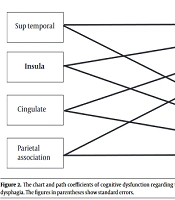
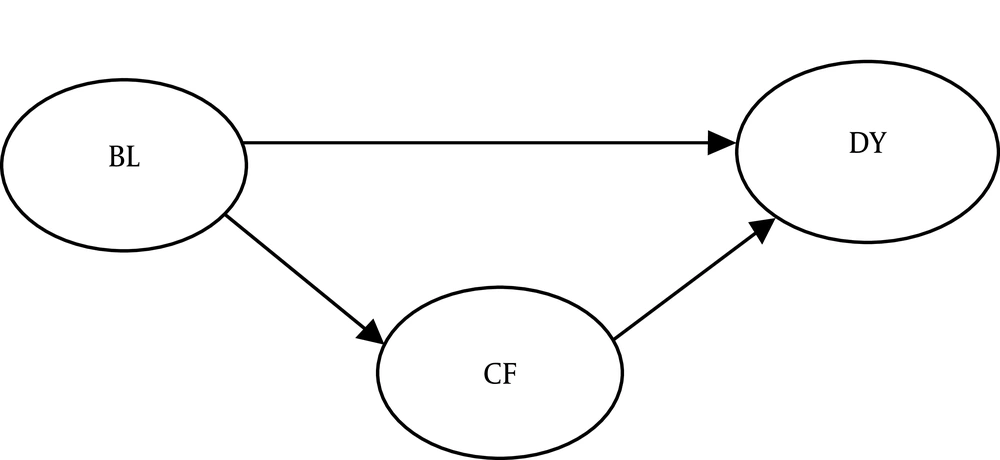
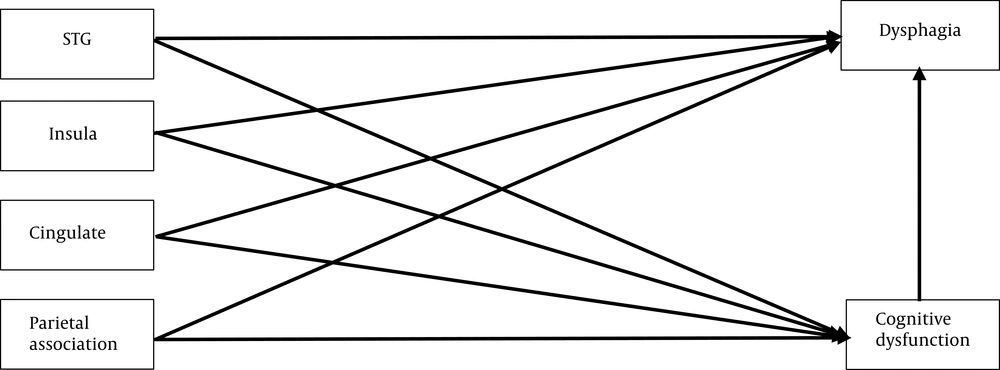
Several studies suggest that brain lesions in the insula, superior temporal gyrus, parietal association cortex, and cingulate gyrus may lead to dysphagia and cognitive disorders (4). Therefore, there are direct relationships between specific brain lesions, dysphagia, and cognitive disorders (Figure 1). Also, several researchers have suggested cognitive disorders as predictors of dysphagia in dementia patients. Moreover, cognitive disorders seem to be associated with delayed swallowing reflexes and reduced tongue movements (4, 8). Therefore, an appropriate model can illustrate the possible relationships between brain lesions, cognitive deficits, and dysphagia.
2. Objectives
It is crucial to identify the role of cognitive function in the development of dysphagia in patients with cognitive disorders, which is the main purpose of this study.
3. Methods
3.1. Study Design, Setting, and Participants
This experimental research will be conducted on individuals with dementia. Patients will be recruited via convenience sampling from the neurology clinics of Shiraz, Iran. Patients with the following criteria will be included in the study: (1) confirmed dementia diagnosed by a neurologist or an expert in the field of neurodegenerative disorders; (2) absence of severe psychiatric or neurological diseases (e.g., stroke within the past 12 months), head injury, visual or auditory agnosia, and acute head or neck diseases; (3) normal or corrected-to-normal vision and hearing; (4) ability to understand instructions and produce/read single words; and (5) signing written informed consent by participants’ parents or caregivers. Using any neuroleptic medication will be recorded and included in statistical analyses.
3.2. Sample Size
The sample size for path analyses should be at least ten times the number of model parameters (9). Therefore, in this study, we will consider a sample size of 30 as the studied model has three parameters. To increase precision, we will increase the sample size to 90.
3.3. Materials
In this study, the brain regions involved in neuroimaging will be detected, and the two variables of dysphagia and cognition will be investigated. We will evaluate dysphagia in two phases. First, all participants with dementia will be assessed based on a screening method called albertinen dementia dysphagia screening (ADDS) that will be used for detecting the risk of aspiration. In this method, a water swallowing test (10, 11) and a test for consistency with different foods (i.e., water, apple puree, and a small slice of apple with a thickness of 1.5 cm) (12) will be combined (10, 12). In the second phase, all known cases of dysphagia, according to ADDS, will undergo a video fluoroscopic examination of aspiration (before, during, and after swallowing).
First, the examiner will check if the continuous X-ray facility is safe to perform the procedure. Then two radiologists, a professor of radiology with 20 years of experience with video fluoroscopic swallowing study (VFSS) and a radiology resident, will conduct the VFSS procedure. We will use a protocol described by Logemann (13). This protocol consists of two trials. The lateral side of the patient’s body will be captured by fluoroscopy in the first trial by asking the patient to sit on a chair and rotate 90 degrees in the opposite direction of the fluoroscope (14). The anterior-posterior side of the patient’s body will be captured in the second trial after asking the patient to sit in an upright position. Throughout the trial, we will ask patients to drink a thin liquid mixture from a cup. In cases with a considerable volume of aspiration, the trial will be terminated, and the patient will be asked to spit out the substance.
All study steps will be taped as AVI files (30 frames/second). Speech therapists with a minimum of five years of experience will interpret VFSS results. The demographic and clinical information of patients will not be disclosed to the interpreters who will only observe patients’ DVD files and report their findings in a standard format. Overall, there are 14 items with weighted values in the video fluoroscopic dysphagia scale (VDS), which show good correlations with aspiration/penetration. These correlations have been observed between VDS and aspiration/penetration only at six months following the onset of dysphagia. The 14 items of VDS show oral and pharyngeal functions, which can be described through VFSS (Appendix 1) (15). Moreover, we will administer the Mini-Mental State Examination (MMSE) test to evaluate patients’ cognitive conditions. Overall, the MMSE is a validated standard test for assessing cognitive impairments in adults (16).
3.4. Procedures
This study will be implemented in the speech therapy department of Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. Patients with dementia will be referred to our department by a neurologist. We will follow four steps in this study. First, all lesions will be localized by a radiologist and entered into a pre-prepared checklist (Appendix 1). Second, the MMSE test will be conducted for each patient separately in a quiet and comfortable room by an expert speech pathologist familiar with the test procedure. In the third and fourth steps, the speech pathologists who have the experience of treating dysphagia will perform ADDS as described earlier and refer positive cases to the radiologist for completing VDS (Appendix 2).
3.5. Data Analysis
A structural equation model will be developed to investigate the relationship between cognition and dysphagia. This model will be implemented instead of segregated regressions to assess the role of cognitive function and investigate a potential causal relationship between the variables in this model (17). A model will be plotted according to the path analysis (Figure 1). Swallowing and cognitive function scores will be calculated and regarded as continuous variables, and the incidence of brain lesions will be treated as a dichotomous item. The weighted least squares mean and variance adjusted (WLSMV) estimation will also be applied to test the path model. The WLSMV will be used in a diagonal weight matrix with robust standard errors and mean- and variance-corrected Chi-square tests (18). An alpha of < 0.05 will be considered for regression coefficients.
4. Results
Moreover, the goodness-of-fit (GoF) index will be measured to evaluate the level of correspondence between the considered models and actual values. The Tucker-Lewis index (TLI), root-mean-square error of approximation (RMSEA), and comparative fit index (CFI) will be used as GoF indices. Overall, the CFI and TLI values of greater than 0.95, as well as RMSEA of smaller than 0.06, represent a good fit between the model and the data (17). SPSS Version 16 will be used to perform these tests. P-value will be set at 0.05 for all statistical analyses.
5. Discussion
The current study mainly aims to investigate the role of cognitive disorders in the occurrence of dysphagia in patients with dementia. The role of cognition in dysphagia development has been suggested in patients with stroke (7). However, as far as we know, the present study is the first one proposing a model for assessing the impact of cognitive dysfunction on dementia. Previous research suggests a relationship between cognitive disorders and dysphagia in patients with dementia (19, 20). However, no study has yet indicated the nature of this relationship. Therefore, clarifying the potential participation of cognitive disorders in the development of dysphagia can provide valuable information for planning dysphagia rehabilitation programs.
The role of cognitive disorders will be evaluated using several models. First, the relationship between cognition and dysphagia in patients with dementia will be discussed separately concerning neuroanatomical lesions. Swallowing and cognitive function are integrated with different neuroanatomical systems, augmenting the risk of various neurodegenerative or vascular disorders. Brain lesions in the insula, superior temporal gyrus, parietal association cortex, and cingulate gyrus can cause dysphagia and cognitive diseases (4). Therefore, if there is an adequate number of patients with these brain lesions, the first model will be presented, involving four separate brain lesion areas (Figure 2). In this model, the role of cognitive disorders will be evaluated in the formation of brain lesions in the mentioned areas. If this model fits the data, dysphagia and cognitive disorder screening will be suggested for all patients with brain lesions in these areas.
In the second model, differences in the performance between various types of dementia (with or without movement disorders) will be considered. In this model, the role of cognitive disorders in the pathogenesis of both types of dementia (with and without movement disorders) will be assessed. If this model fits the data, dysphagia without movement disorder will be the most significant finding of our analysis. On the other hand, dysphagia rehabilitation programs for dementia patients without movement disorders will be focused on cognitive therapy, which has been neglected in current dysphagia rehabilitation programs. Moreover, for patients with dementia and movement disorders, if the model fits the data, rehabilitation programs will be suggested to be performed simultaneously for both the movement and cognitive disorders. Overall, purposeful rehabilitation programs can help us to more efficiently achieve our short-term goals.
Considering the total MMSE score, as well as the scores of its three domains (i.e., attention, executive function, and memory) involved in swallowing (21), a third model will be used to evaluate these three cognitive domains. If the third model fits the data, cognitive rehabilitation will provide us the best swallowing outcomes in dementia patients with dysphagia.
Finally, the implications of this study, such as shedding light on the mechanisms of dysphagia development in patients with dementia, will be described. The fitness of the suggested models is crucial to indicate the importance of cognitive therapy in managing dementia patients with dysphagia. In the third model, the role of cognitive disorders will be evaluated by examining different cognitive domains involved in swallowing. If the third model fits the data, cognitive rehabilitation will offer the best cognitive and swallowing outcomes in dementia patients with dysphagia. Overall, during video-fluoroscopy, there is a need for patients to greatly cooperate, and one of the anticipated limitations of this study may be dementia patients’ inadequate cooperation.