1. Background
Chronic stress or glucocorticoids exposure for a long time causes negative changes in hypothalamic-pituitary-adrenal axis (HPA axis), which leads to morphological and functional alterations in the hippocampus. Based on previous studies, these alterations are very vulnerable to the neurotoxicity caused by stress (1). Those changes include modifications in dendrite outgrowth and spines that eventually lead to their loss, and consequently, reduction in the hippocampal synaptic plasticity and volume and the suppression of neurogenesis. These are the common changes observed following chronic stress in rodents and patients with major depression (2). Brain-derived neurotrophic factor (BDNF) is a neurotrophin abundant in the hippocampus that plays a significant role in neurogenesis, promotion of synaptic plasticity, and neural cell survival, which may all lead to proper levels of cognitive ability, like learning and memory (3). According to previous studies, chronic stress and depression are linked to the reduction of BDNF production and receptor activity in the hippocampus (4, 5).
Studies have demonstrated that 1,25(OH)2 vitamin D3 or vitamin D exerts critical effects on brain development and function, in addition to other neuroprotective effects, including anti-inflammatory (6) and anti-oxidative effects (7). Vitamin D also promotes the expression of neurotrophic factors, such as glial-derived neurotrophic factor (GDNF) and neurotrophic factor -3 (NT3), particularly, in the hippocampus (8). Previous studies have also shown the modulatory effect of vitamin D on glucocorticoid production and activity (9, 10). Furthermore, the cross-talk between glucocorticoids and Vitamin D receptors exists in various regions of the brain (11).
Regarding the negative effects of chronic stress on the structure and function of the hippocampus and neuroprotective roles of vitamin D, we conducted this experiment to understand the protective effect of vitamin D on the hippocampal BDNF levels in a restraint model of chronic stress, since BDNF levels is a good indicator of the integrity of the hippocampal synaptic function (4, 5). Therefore, the results of this experiment may underscore the significance of this vitamin in protecting the cognitive ability against the disabling effect of daily chronic stress on the nervous system.
Few studies have explored the protective role of vitamin D against the negative effects of chronic stress on BDNF protein expression and some other neurotrophic factors (10). Therefore, in this study we used restraint stress as a model of chronic stress in rodents to evaluate the protective effect of vitamin D first on serum corticosterone level, as an indicator of HPA axis disruption, and then on BDNF level in the hippocampus. Moreover, we used 3 hours of restraining for 28 days versus 6 hours per day for 21 days, which a more common type in stress studies. We demonstrated that weaker stress induction to reduce animal suffering is equally reliable as a stronger model of chronic stress in altering CORT and BDNF levels in the rats.
2. Methods
Male Wistar rats (n = 60) (Research Centre of Physiology, Semnan University of Medical Sciences, SUMS, Semnan, Iran) weighing 170 - 200 g were used in this experiment. Food and water were available ad libitum. All experiments were conducted between 08.00 - 12.00 h. The rats were weighed before the experiments and then weekly throughout the study. All the experimental procedures were carried out under the agreement of the Animal Care and Use Committee of SUMS (code of ethics: IR.SEMUMS.REC.1396.189).
Vitamin-D3 (Iran Hormone Co., Tehran, Iran) was dissolved in 100% ethanol (1 μg/μL, stock) and diluted in 5% ethanol (vehicle) for intraperitoneal (i.p.) injection. Vitamin D was used in two concentrations of 5 and 10 μg/kg (12-14), twice weekly.
All the animals were randomly divided into six groups, each containing 10 rats (n = 10). The groups were organized based on the type of treatment: (1) non-stressed /vehicle, (2, 3) non-stressed /Vitamin D (5μg/kg or 10 μg/kg), (4) stressed /vehicle, (5, 6) stressed /Vitamin D (5 μg/kg or 10 μg/kg).
Restraint stress was exerted by placing the rat in a plexiglass restrainter from 09.00 am to 12.00 pm every day (15) for 28 days. After the stress procedure, the rat was returned to the home cage.
On the 29th day, blood was collected by cardiac puncture under anaesthesia (Ketamine 40 mg/Kg and Xylazine 5 mg/Kg), then rats were sacrificed by decapitation, and the hippocampus was dissected over the ice, stored in a microtube, and kept in (-80°C) a freezer until use.
Serum CORT levels of all the groups were measured by the rat CORT ELISA kit (Hangzhou East Biopharm Co., Ltd, China). ELISA was carried out between 08:00 - 12:00 h based on the manufacturer’s instructions.
For BDNF assay, the hippocampus was homogenized in lysis buffer (100 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% TritonX-100, 5% sodium deoxycholate with protein inhibitor cocktail, 13911-1BO, Sigma, added). Protein concentration was measured by the Bradford method. After SDS-Page, proteins were transferred to poly vinylidene disulforide (PVDF) membrane and incubated with primary antibodies BDNF (1:500, PA1-18371 Invitrogen, USA) and GAPDH (1:1000, SC-25778 Santa Cruz, USA). The samples were then incubated with secondary antibody (HRP-conjugated goat anti-rabbit IgG; 1:2000, Sigma Aldrich). Later, membranes were incubated with DAB, as a chromogenic detecting substrate, and the optical density of each band was measured by ImageJ software (NIH, USA).
2.1. Statistical Analysis
Two-way analysis of variances (ANOVA) and pair-wise comparison (Sidak’s multiple comparison test) were carried out by GraphPad Prism 8.0.2 (GraphPad Software, 2019). Data is presented as mean ± SEM. A P-value of less than 0.05 is considered statistically significant.
3. Results
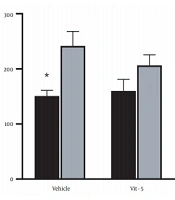
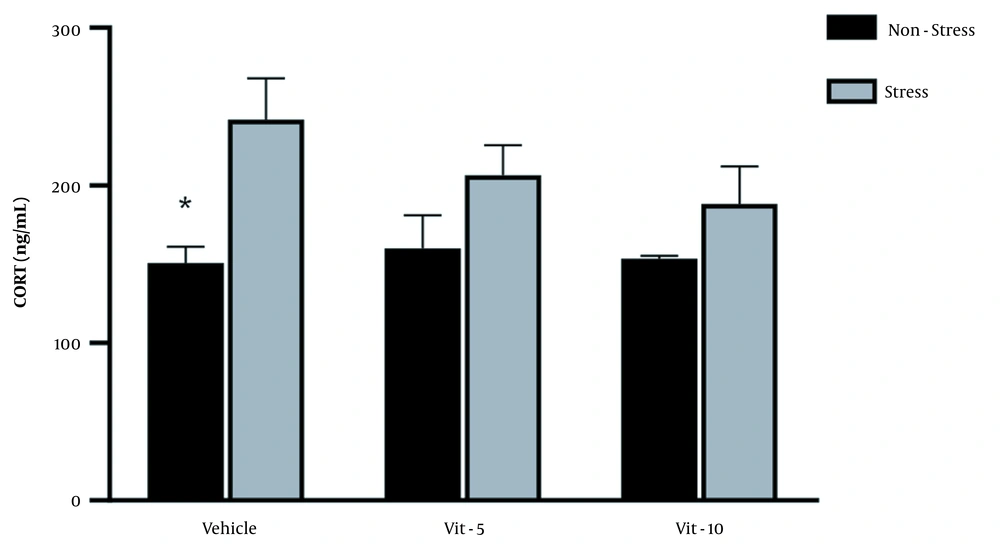
The comparison of mean (± SEM) CORT levels revealed that restraint stress caused a significant increase in the CORT levels in the stress group versus the non-stress group (F (1, 27) = 7.967, P = 0.0088), but vitamin D/vehicle treatment did not produce any marked difference in the serum CORT levels in the restraint stress-treated rats. Also, the interaction between stress treatment and vitamin D/vehicle treatments was not significant. Sidak’s multiple comparison test revealed a significant rise in the CORT level of stress /vehicle group versus the non-stress /vehicle group (P < 0.05), indicating that HPA axis activity was affected by stress (Figure 1).
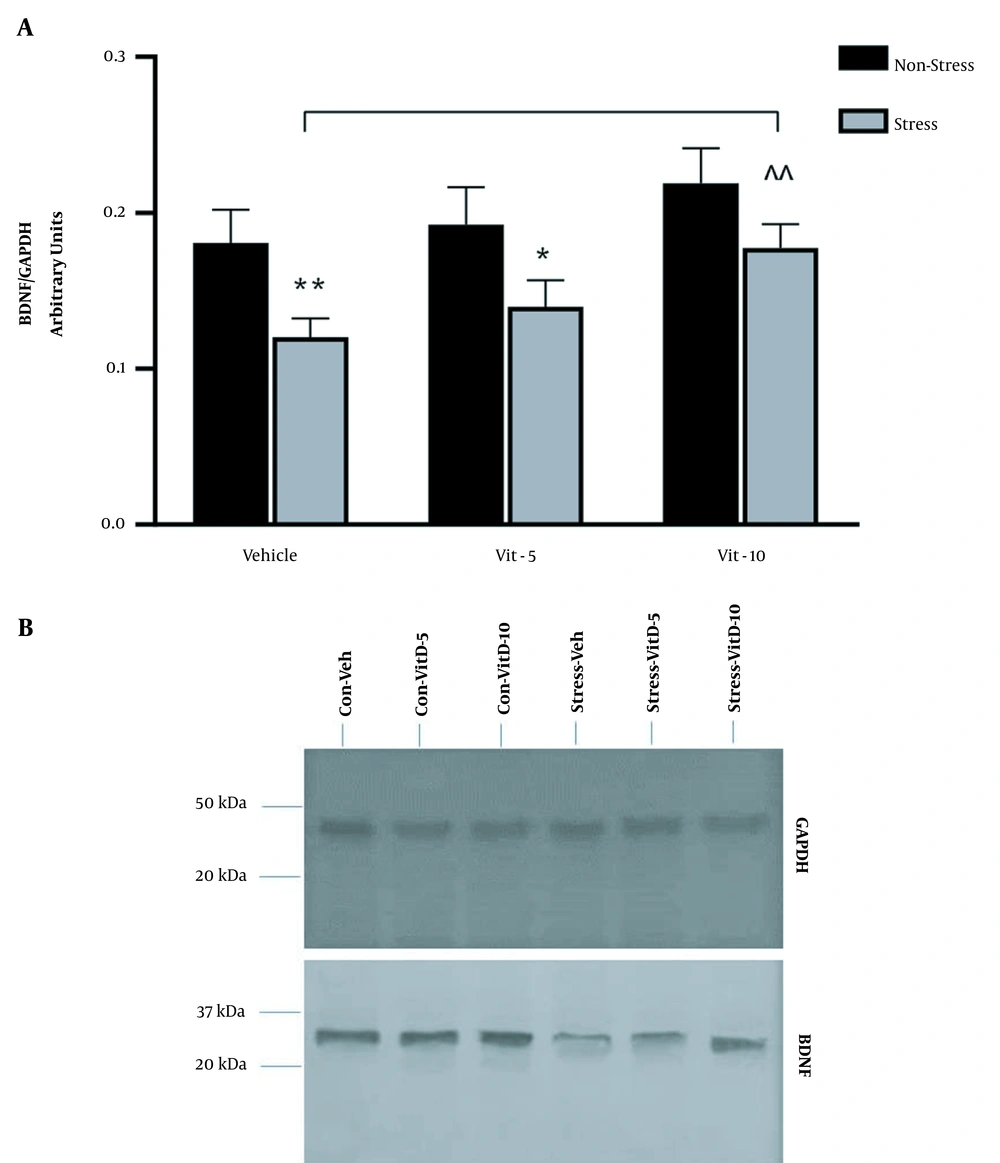
Measuring the BDNF protein mean (± SEM) levels in the hippocampus revealed a significant increase in the BDNF levels of the stress group versus the non-stress group (F (1, 12) = 32.92, P < 0.001) and between treatments (F (2, 12) = 9.720, P = 0.0031), but the interaction between groups and treatments was not significant. Sidak’s multiple comparison test revealed that stress significantly reduced BDNF levels in the stress-treated group compared to the non-stress /vehicle group (P < 0.01). Vitamin D-5 raised BDNF level in the hippocampus, although it was still significantly lower than the non-stress/vitD-5 group (P < 0.05). However, vitamin D-10 increased BDNF to the level that it was not anymore significantly different from that in the non-stress/vitD-10 group, and it also raised BDNF levels higher than stress/vehicle group (P < 0.01; Figure 2A). The lack of difference between the non-stress and stress vitD-10 groups, along with marked difference between vitD-10 and vehicle-treated groups suggests that vitD-10 might have successfully kept BDNF levels close to normal against the negative effects of stress on BDNF expression in the hippocampus.
Ratio of brain-derived neurotrophic factor (BDNF) protein level to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the hippocampal tissue of restraint stress and non-stress rat brains. (A) Restraint stress suppressed BDNF protein level relative to the non-stress rats (* P < 0.05, **P < 0.01). Treating restraint stress rats with vitamin D increased BDNF level. Vitamin D (10 μg/kg) significantly increased BDNF level in stressed rats relative to vehicle-treated stressed rats (^^P < 0.01). (B) BDNF protein bands in all the six groups (3 non-stress and 3 restraint stress) are shown as a normalized ratio to GAPDH protein level.
4. Discussion
In this study, we demonstrated that 3 hours/day of restraint stress on rats for 28 days increased the serum CORT level while receiving a higher concentration of vitamin D during the stress treatment prevented the rise of CORT level. Previous studies indicated that various forms of repeated and chronic restraint, such as chronic mild stress (CMS), could elevate CORT levels in rats due to the activation of HPA axis (10, 16-18). Also, previous studies have reported the significant impact of vitamin D on reducing elevated CORT levels due to stress in ovariectomized rats or decrease glucocorticoid passage through the placenta in pregnant mice (10, 19).
In contrast to those studies that used 6 hours of restraining per day as a model of induction of chronic stress (16), in this study, we did not observe a significant reduction in the CORT levels of rats under vitamin D treatment; however, treatment with vitamin D reduced the significant difference in BDNF levels between the stressed and non-stressed hippocampus. The difference observed in study results might be related to the shorter duration of stress per day (not the length of stress period). If, however, vitamin D reduces serum CORT level significantly, it might possibility be because of its interference with glucocorticoid production in the adrenal cortex through its action on the vitamin D response element on the promoter of CYP21A2, the gene that encodes 21-hydroxylase enzyme, indicating that the active vitamin D (1,25(OH2) D3) can downregulate the expression of 21-hydroxylase, which is required for glucocorticoids and mineralocorticoids synthesis (20, 21). The other possibility might be because of the central effect of vitamin D on glucocorticoid regulation. In a study conducted by Koshkina et al. in 2019, they showed that vitamin D decreased ACTH and corticosterone level in ovariectomized rats that underwent chronic unpredictable mild stress (CUMS) (10). Therefore, vitamin D could act on vitamin D receptor, VDR, in the hypothalamus to control CRH production, which might occur under stronger stress induction rather than what we used in this study.
In this study, we also measured the BDNF protein levels in the hippocampus of stressed and non-stressed rats. Restraint stress markedly lowered BDNF protein expression in rats. Treating rats with vitamin D restored BDNF close to the normal levels. These data indicates that vitamin D may have opposed the stress effects on BDNF expression in the hippocampus. Delirious effects of stress on the hippocampus structure and neurotrophic factor expressions and decrease in BDNF mRNA expression in relation to different types of chronic stress models, particularly, the restraint stress, has been described in details previously (4, 5, 22). On the other hand, treating cultured neural stem cells from the rat hippocampus with the active form of vitamin D has been shown to upgrade BDNF mRNA levels (9).
In another study, exposing the hippocampus progenitor cell line to dexamethasone (Dex) inhibited the neurite outgrowth, while treating them with vitamin D significantly reduced Dex effects (23). Likewise, in an in vivo study, vitamin D increased BDNF protein in obese rats with a high-fat diet and improved their cognitive abilities (24). Also, maternal vitamin D deficiency changed fetal brain development because of the alterations in the expression of BDNF and transforming growth factor (25). Likewise, in a recent study, vitamin D effectively resolved post-stroke depression in mice by elevating hippocampal BDNF levels (26). However, the infusion of the active form of vitamin D3 directly into the hippocampus of rats markedly reduced BDNF levels, which was previously increased by physical exercise (27). It is most likely that vitamin D has specific regulatory effects on the neural structures involved in stress or inflammation, but not at normal physiological conditions.
In contrast to CUMS that has been widely used in various studies, including the evaluation of vitamin D function in the brain, restraint stress has not been utilized that frequently in vitamin D studies (except for few studies using restraint stress during various time frames for inducing PTSD and studying the changes in the hippocampal VDR (28)). In this study, we demonstrated that restraint stress (3h/day for 28 days) was able to increase serum CORT and decrease hippocampus BDNF levels, similar to the stronger type with 6h/day for 21 days of restraining.
An interesting finding in this study was that although vitamin D did not reduce serum CORT levels significantly, but it markedly increased BDNF levels in the hippocampus. This finding is somehow different from some previous studies (7) since they demonstrated marked reduction in CORT by vitamin D along with increase in BDNF levels in the brain. Our study suggests that vitamin D may modify the hippocampal BDNF levels through ways not directly related to the HPA axis, even under chronic stress.
4.1. Conclusions
Since limited studies have so far investigated the neuroprotective effect of vitamin D in relation to chronic stress neural system damages, this work was conducted and demonstrated that applying restraint stress increases serum CORT (HPA axis function indicator) and lowers the hippocampal BDNF levels. However, high concentrations of vitamin D maintained the hippocampal BDNF protein levels close to control. This study also indicates that vitamin D may interfere with the HPA axis, but possibly regulate the hippocampal BDNF expression level through various pathways in chronic stress. This study also points to the protective role of this vitamin in promoting the cognitive ability, learning, and memory process. Nevertheless, further studies are required to elucidate the unclear mechanism of action of this vitamin in relation to glucocorticoid system and hippocampal BDNF regulation.