1. Background
Stroke results of the sudden cessation of blood flow to the brain, leading to neuronal death, loss of sensorimotor, and cognitive and memory dysfunctions (1). A large part of focal ischemic strokes is due to middle cerebral artery occlusion (MCAO), causing damage to the hippocampus, loss of spatial memory, and cognitive dysfunction (1, 2). However, poststroke treatment is currently very difficult and limited to thrombolytic and thrombectomy therapies (1). Therefore, preventing stroke attacks in susceptible patients is very important. Patients with transient ischemic stroke, atrial fibrillation, and asymptomatic carotid stenosis are at high risk for stroke attacks (3); therefore, the use of appropriate drugs as a prophylaxis approach can be useful in preventing stroke attacks or minimizing the damage after the occurrence of cerebral ischemia in these groups.
Chemerin (a new adipokine) is a multi-potential peptide mainly synthesized and released from adipose tissue and plays an important role in inflammation, glucose and lipid homeostasis, angiogenesis, and energy balance (4). It has been found that chemerin may be associated with the pathogenesis of a number of diseases in humans, including metabolic syndrome, diabetes, atherosclerosis, and cardiovascular diseases (4, 5). Moreover, preclinical evidence has recently indicated that post-ischemic treatment with chemerin protects neurons against hypoxia and cerebral hemorrhage in neonatal rodents when administered intranasally by inhibiting pro-inflammatory cytokines and neuronal apoptosis (6, 7). It has also been reported that chemerin receptors are highly expressed in the prefrontal cortex, hippocampus, and hypothalamus in rodents (8, 9). We have also recently found that post-ischemic intervention of recombinant human chemerin (rh-chemerin) reduces brain damage via suppressing the anti-inflammatory and apoptotic pathway in an experimental model of stroke in mice (10). Nevertheless, the prophylactic effect of rh-chemerin in the experimental model of stroke is not clear.
2. Objectives
It is unclear whether pretreatment with rh-chemerin can diminish injury after a stroke attack in mice. Therefore, this work was designed to examine the effects of the pre-ischemic intervention of rh-chemerin on brain injury and spatial memory disturbance in a mouse model of stroke.
3. Methods
3.1. Animal and Drug
Adult male Swiss albino mice (30 - 40 g, 3 - 4 months old) were provided from the animal house of Semnan University of Medical Sciences (SUMS). All protocols performed in this study were in accordance with Ethical Guidelines for the Use of Animals in Research. To reduce surgical pain, buprenorphine (Temad Co, Active Pharmaceutical Ingredients, Iran) was given intraperitoneally half an hour before and 8 hours after surgery.
Recombinant human chemerin (Sigma-Aldrich, SRP6002, USA) or saline as a vehicle (10 µL) was slowly injected into the bilateral nostrils by a PE10 catheter within 30 seconds.
3.2. Focal Cerebral Ischemia
Transient models of focal cerebral ischemia were established in mice (11). Briefly, under chloral hydrate (400 mg/kg, intraperitoneally) and laser Doppler flowmetry (LDF; Moor Instruments DRT4, UK) monitoring, MCA was blocked using a silicone-coated 7 - 0 monofilament for 60 minutes, and then reperfusion was made for 23 hours. For continuous monitoring of cerebral perfusion 15 minutes before, during 60 minutes of MCAO, and up to 15 minutes after reperfusion, a needle probe of LDF (DP3, Moor Instruments, UK) was fixed on the surface of the right temporal bone using a probe holder.
3.3. Experimental Protocols and Design
To study the prophylactic influence of intranasal rh-chemerin (800 ng/mouse) on lesion size, spatial memory, and neurological function, 23 mice were randomly divided into 3 groups, including the sham-operated group (surgery + no MCAO; n = 7), control ischemic group (MCAO + saline; n = 8), and treatment group (MCAO + 800 ng/mouse rh-chemerin; n = 8). Animals received saline as a vehicle (10 µL, intranasally) and/or rh-chemerin daily for 7 days prior to ischemia. The last dose of rh-chemerin was prescribed 30 minutes before MCAO on the seventh day of the experiment. According to our previous study, the dose of 800 ng/mouse of rh-chemerin was chosen as the therapeutic dose (10). Neurological and spatial memory functions were verified, and then animals were sacrificed. Infarct sizes were measured 24 hours after MCAO.
3.4. Neurobehavioral Examination
Neurological tests (including motor and sensory examinations) were performed in accordance with neurological deficit scores classified as follow: (1) severe: 10 - 14; (2) moderate: 5 - 9; and (3) mild: 1 - 4 (12).
3.5. Spatial Learning and Memory Testing
Spatial learning and memory were accessed using a radial arm water maze (RAWM) apparatus with 6 arms (length = 45 cm, height = 10 cm, and diameter = 10 cm) in a swimming pool and buried platform, which was placed at the end of the target arm (10, 13). RAWM experiments were performed in 3 phases: (1) habituation (day 1): at this stage, the animals for 2 - 3 minutes were acquainted with RAWM; (2) learning period (days 2 - 5): the animals received 5 experiments per day with an interval of 30 seconds between experiments for 4 consecutive days to find a buried platform. The animals had 60 seconds to find a covered platform underwater. If the animal could not detect the position of the platform, it was helped to move toward the platform. They were then allowed to remain on the platform for 30 seconds; (3) memory testing (day 6): at this stage, the animals were anesthetized, and cerebral ischemia was induced by the conclusion of MCA. Then, 24 hours after ischemia, to perform the probe test, the animal was allowed to detect the position of the removed platform for 60 seconds. The time consumed in the platform location and the target region were evaluated (Noldus EthoVision XT7, the Netherlands). Animals with severe motor deficits for swimming were excluded from the study.
3.6. Infarct Size
2,3,5-Triphenyltetrazolium chloride (2% TTC) staining (T8877, Sigma, Germany) method was used to determine the infarct area. Briefly, the animals were sacrificed under deep anesthesia, the brain was removed, and then sections with 2 mm thickness were achieved. Brain slices were stained and photographed. The infarct area (white zone) was measured using an image analysis software package (Scion Image version 4.0). Finally, the volume of infarct size was obtained by multiplying the white zone in the thickness of each section. The results were presented as infarct volume (mm³) normalized for edema (14).
3.7. Data Analyses
The Shapiro-Wilk test was used to check the normality test of data. One-way analysis of variance (ANOVA), followed by the post hoc Tukey test, was used to statistical analyses data [SigmaPlot 12 (2012), Systat Software Inc]. Data were demonstrated as mean ± SEM. P < 0.05 was considered statistically significant.
4. Results
4.1. Cerebral Blood Flow
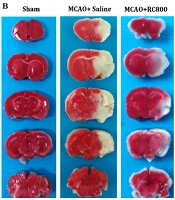
Regional cerebral blood flow was recorded by LDF 15 minutes before, during 60 minutes of MCAO, and up to 15 minutes after reperfusion. Ischemia was confirmed in the control and treatment groups with a reduction in blood flow below 20% of baseline (Figure 1A). No significant difference was observed between the control and treatment groups during 60 minutes of ischemia and 15 minutes of reperfusion (P > 0.05; Figure 1A).
A, Local cerebral blood flow; B, 2,3,5-Triphenyltetrazolium chloride staining image; C, Infarct volume; and D, Neurological deficit scores in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 [middle cerebral artery occlusion + recombinant human chemerin (800)]. Values are presented as mean ± SEM (n = 7, each group) (*P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group).
4.1. Effect of Pre-ischemic Treatment of Recombinant Human Chemerin on the Infarct Size and Neurological Outcome
After 60-minute ischemic and 23-hour reperfusion, the infarct size was 125 ± 4 mm3 in the control group (MCAO + saline). Pre-ischemic administration of rh-chemerin for 7 days noticeably diminished the lesion size (56 ± 5 mm3) compared to the MCAO + saline (control) group (P < 0.001; Figure 1B and C). Moreover, pretreatment with rh-chemerin significantly improved neurological outcomes (P > 0.05; Figure 1D).
In the control group (MCAO + saline), 60% of mice died (12 out of 20), and in the treated group (MCAO + rh-chemerin), about 38% of mice died (5 out of 13) before the end of the experiment (they were excluded from the study). In the sham group, the mortality rate was zero.
4.3. Effect of Pre-ischemic Treatment of Recombinant Human Chemerin on the Spatial Learning and Memory
The RAWM test showed that after 4 days of training, escape latency time to find the location of the platform was considerably shorter (P < 0.01; Figure 2A). This showed that learning in all 3 groups was done correctly. After cerebral ischemia, time to find the location of the platform significantly increased and also time spent in the location of target zone reduced (P < 0.01; Figure 2B and C). Following pre-ischemic treatment with rh-chemerin, time to find platform location significantly reduced, and time spent in the target zone increased (P < 0.01; Figure 2B and C).
A, Escape latency (second) during 4 days of training; B, Time to find the platform location; and C, Time spent in the target zone during the probe test in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 (middle cerebral artery occlusion + recombinant human chemerin [800]). Values are presented as mean ± SEM (n = 7, each group) (P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group).
5. Discussion
Our findings indicated that pretreatment with rh-chemerin for 7 days prior to ischemia reduced the infarct volume by 56% compared to MCAO + saline as the ischemic control group. These data are consistent with previous preclinical studies describing that rh-chemerin protected neuronal damage against hypoxic-ischemic encephalopathy and germinal matrix hemorrhage in neonatal rats (6, 7). Moreover, the mortality rate was 60% in the control group, which was reduced to 38% after per-ischemic treatment with rh-chemerin. This suggests that the reduction in mortality rate after intervention with rh-chemerin is probably due to reduced brain damage.
The mechanisms through which rh-chemerin reduced brain damage were not examined in this study. However, it has been well documented that activation of inflammatory responses and programmed cell death after stroke can increase primary brain damage; thus, inhibiting this pathway can stop or decline the development of primary injury (15). Recent experimental studies have shown that rh-chemerin has anti-apoptotic and anti-inflammatory effects in hypoxia-ischemia conditions (6, 7). Moreover, our recent research showed that post-ischemic treatment with rh-chemerin diminished stroke that induced injury by inhibiting pro-inflammatory cytokines and the machinery of programmed cell death (10). Therefore, the protective effects of rh-chemerin in this study may be attributed to these effects. However, further studies are needed to find mechanisms of neuroprotection of rh-chemerin in stroke.
Cognitive and sensorimotor impairments are the most common complications of stroke, affecting patients’ quality of life (16). Cognitive impairments after stroke include dysfunction in attention, memory, language, and orientation (16). Previous studies have reported that more than 50% of stroke patients suffer from sensory-motor and memory disorders (16, 17). In this study, spatial memory was evaluated by the RAWM apparatus in mice. Our result indicated that intranasal pre-ischemic treatment with rh-chemerin for 7 days significantly reduced spatial memory loss in mice. In this regard, previous studies have consistently reported that treatment with chemerin increases cognitive performance in animals with hypoxic-ischemic encephalopathy (6) and mouse models of stork (10).
Neurological examinations after stroke are important in assessing the condition of stroke patients. In addition to brain imaging, post-stroke neurological tests can be useful in identifying the severity of brain damage or responding to medication. The current study showed that pre-ischemic administration of rh-chemerin (800 ng/mouse) corrected neurological disturbance. This finding is consistent with previous studies showing that treatment with rh-chemerin reverses neurological dysfunction in germinal matrix hemorrhage and hypoxic-ischemic encephalopathy in neonatal rats (6, 7). Moreover, our recent study demonstrated that post-ischemic administration of rh-chemerin significantly reduced neurological disorders in mouse models of stroke (10), confirming the results of the current study.
5.1. Conclusions
Our findings for the first time revealed that intranasal administration of rh-chemerin for 7 days before ischemia reduced brain damage and improved neurological and spatial memory functions in a mouse model of stroke. This finding may be useful for patients at risk for stroke attacks. However, further studies are needed on the use of rh-chemerin as a prophylaxis approach in high-risk people.
![A, Local cerebral blood flow; B, 2,3,5-Triphenyltetrazolium chloride staining image; C, Infarct volume; and D, Neurological deficit scores in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 [middle cerebral artery occlusion + recombinant human chemerin (800)]. Values are presented as mean ± SEM (n = 7, each group) (*P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group). A, Local cerebral blood flow; B, 2,3,5-Triphenyltetrazolium chloride staining image; C, Infarct volume; and D, Neurological deficit scores in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 [middle cerebral artery occlusion + recombinant human chemerin (800)]. Values are presented as mean ± SEM (n = 7, each group) (*P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group).](https://services.brieflands.com/cdn/serve/3170b/f53be5ec01d2a72cb749f05ebb16ecdae8fb3695/mejrh-120354-i001-F1-preview.webp)
![A, Escape latency (second) during 4 days of training; B, Time to find the platform location; and C, Time spent in the target zone during the probe test in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 (middle cerebral artery occlusion + recombinant human chemerin [800]). Values are presented as mean ± SEM (n = 7, each group) (P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group). A, Escape latency (second) during 4 days of training; B, Time to find the platform location; and C, Time spent in the target zone during the probe test in the sham-operated (surgery + no middle cerebral artery occlusion), control ischemia (middle cerebral artery occlusion + saline) and recombinant human chemerin treated groups at a dose of 800 (middle cerebral artery occlusion + recombinant human chemerin [800]). Values are presented as mean ± SEM (n = 7, each group) (P < 0.001 compared to the middle cerebral artery occlusion + saline group; #P < 0.001 compared to the respective sham group).](https://services.brieflands.com/cdn/serve/3170b/91451ab315b58693f82fab887244be59c363cab6/mejrh-120354-i002-F2-preview.webp)