1. Background
With the increasing population, the improvement of living standards, followed by the increase in water consumption and the decrease in water resources, and the increase in physical, chemical, and microbial pollution of water, the water crisis has become one of the major global problems. The most water-related concerns are in arid and semi-arid regions, and more than 80% of diseases worldwide are water-based diseases (1).
According to the Centers for Disease Control (CDC), the nosocomial incidence of legionellosis is between 25 and 45% in the environment, and the rate of mortality due to the disease is 30% in hospital cases; some sources has been reported more than 40% rate, gender, smoking, consumption of alcohol, underlying diseases such as chronic lung disease, cardiac diseases, type 2 diabetes, inadequate antibiotic treatment, impaired immunity, long-term hospitalization, and kidney transplantation are factors affecting the disease risk (2). Among these causes, smoking and chronic lung disease are the most common. The World Health Organization reports the Drinking Water Quality Index annually. The average disease load index (one person in year) is equal to 10-6. Accordingly, the risk of infection and mortality from Legionella should be around 10-4 and 10-7, respectively. Legionella reference values in the Drinking Water Quality Guide are 0 CFU/100 mL, equivalent to 1 CFU/L (2).
France, Italy, and most European countries are at risk of water contamination by Legionella bacteria, determined it as 10,000 CFU/L in the distribution network, and emphasized that if the pollution goes higher than this amount, control measures are necessary. A review of the results of studies shows Legionella bacteria in hospital water sources, hotels, swimming pools, other health facilities, temporary accommodation, residential homes, workplaces (offices and factories), natural water resources, and man (2). Also, according to the World Health Organization, 2.4 million out of four billion cases of diarrhea are due to a lack of access to safe drinking water (3). Every day, 50,000 people worldwide die from water-borne diseases, many of which occur in developing countries that do not have access to adequate water resources (4).
Improving public health without achieving safe drinking water is not possible. More than 250 million people are infected with water-related diseases each year, leading to five to 10 million deaths a year, well above the DALYs. The CDC reports that 1,020 people in the United States became infected with water-borne diseases in 2001 and 2002. Microbial diseases are one of the most important water-borne diseases in humans. These contaminants may occur directly from drinking contaminated water or indirect contact (5).
After entering the respiratory system, Legionella can survive and proliferate inside host cells and reach the target cells mainly by inhalation and aspiration (6). The bacterium is also capable of voluntary intracellular life and can grow and multiply in pulmonary monocytes, macrophages, and the cytoplasm of some protozoa such as free amoebae, Acanthamoeba, and Naegleria. After Legionella pneumophila enters the respiratory system, it attaches to epithelial cells. The organism's resistance inside the alveolar macrophages plays an essential role in the progression of the disease.
Of 50 identified species and 70 serogroups of Legionella, approximately 20 species are pathogenic to humans (7). Legionella pneumophila has 16 different serogroups (8), which cause legionellosis in more than 90% of cases. Legionella pneumophila serogroup (SG1) is the cause of 84% of epidemics and recurrences (9). This bacterium is replaced in the human respiratory tract by inhaling small water particles carrying the bacterium, leading to infection (6, 10, 11).
According to the 2018 study of subgroups, the prevalence rates of Legionella spp. in hospital water, dental settings water, and other water resources were 28.8%, 23.6%, and 29.6%, respectively. The most common species of Legionella was related to L. pneumophila with a prevalence of 60.5%, while the prevalence of all other species was 52.5%. Based on location, the highest prevalence was reported in Isfahan with a rate of 55.7%, followed by Tehran with a prevalence of 27.9% (10).
People with immunodeficiency, organ-receiving patients, the elderly, alcoholics, and drug addicts are more likely to develop the nosocomial infection. Legionella species cause two types of independent or clinical diseases, including legionellosis and Pontiac fever (a self-limiting disease similar to influenza) (10, 12). This bacterium is found naturally in lakes, rivers, hot springs, swimming pools, water pipes, cooling towers, and ventilation systems (8, 13). Water distribution networks in hospitals are an important source of Legionnaires' disease (14). This bacterium is a water flora and can survive in waters with different temperatures (0 - 68°C). It has a pH of 8 - 5, and hot water systems are an ideal environment for the growth of this bacterium (15, 16). The coexistence of Legionella bacteria with algae and other bacteria, especially in the biofilm complex, as well as protozoa, provides the necessary food sources for bacterial survival (17). Temperature and nutrition have a significant effect. Legionella pneumophila has resistance and distribution in aqueous environments, and this bacterium survives for a long time due to its metabolic activity in low-temperature waters (18).
Aquatic biofilms provide special ecological conditions for the growth and reproduction of Legionella. High temperatures, organic and inorganic substances in water, and the presence of protozoa have a significant impact on their growth and spread. Some substances, such as plastic faucet gaskets, increase the growth of Legionella. Bacteria such as Aeromonas, Mycobacterium, Pseudomonas, and Flavobacterium species, especially in the biofilm complex, provide protection and conditions for the growth and survival of bacteria. This relationship creates conditions for the bacterium to easily resist adverse environmental conditions and disinfection (19, 20).
Due to the health problems caused by Legionella and the fact that water in various centers and places is the most important cause of disease transmission, it is necessary to take adequate measures to remove this bacterium from water sources, especially in medical centers. The studies have increased knowledge about Legionella infection. Due to the role of this bacterium in nosocomial infections and mortality in high-risk patients, planning is essential to identify infected sources and control them.
The sample selection criteria were based on the claim of George Fisher, stating that its pipes and fittings have anti-legionella properties. Also, the RayHo and metal piping systems were used for comparison.
2. Objectives
This study aimed to investigate the effect of Legionella stopper pipes and fittings in George Fisher Company for controlling Legionella growth in the indoor water supply system.
3. Methods
This descriptive cross-sectional study was conducted for three months (from the beginning of autumn to the end of autumn 2020) in the water supply system of two commercial centers with three different types of piping (George Fischer pipes and fittings, RayHo pipe, and ordinary polyethylene pipes). The G1 center used George Fischer Legionella stopper pipes and fittings for indoor water supply. The G2 center used RayHo pipes and fittings. Center 3 had regular polyethylene pipes. All three centers were located close to each other. Then, we collected 56 samples (36 samples of cold water and 20 samples of hot water) from the gallery-commercial center of Galleria, including 34 samples of the system with pipes and fittings of George Fischer and 22 samples of the ward equipped with pipes and fittings of Ray Ho. Also, five samples of Taleghani hospital were collected with a conventional metal piping system in sterile containers made of polyethylene with a volume of 1.5 liters from different parts of the office-commercial complex of Gallery and Taleghani hospital. The samples were analyzed for the presence of Legionella.
We used 3% sodium thiosulfate to remove residual chlorine from water samples. Residual chlorine, pH, and temperature were measured at the sampling site according to standard methods. Samples were immediately transferred to the laboratory in the vicinity of ice boxes for a maximum of eight hours and concentrated using membrane filtration and vacuum pumps (model VE115N) and polycarbonate micron carbon filters (Uflow Membrane Filters, pore size: 0.22 - 0.45 μm). After filtration, the filter was separated and 50 ml filtered water was poured into sterile tubes and then cultured on BCYE medium.
3.1. Culture
The BCYE agar medium (Biomark) containing L-cysteine supplements (Merck, Germany), pyrophosphate (Sigma Aldrich), and GVPC (glycine, vancomycin, polymyxin B, cycloheximide) (Biomark) was used for bacterial culture according to the instructions. To ensure the control of other bacteria, the samples were heat-treated using a water bath (56°C for 12 minutes) before culture. Then, 100 μL of the sample was inoculated on the culture medium, and the plates were placed in a candle jar containing 2 - 5% carbon dioxide for 7 - 14 days at 37°C in a humid environment (Figure 1). Legionella colonies were identified based on size, color, and biochemical properties (catalase, oxidase, hydrolyze Hippurate, and Gram staining tests). The grown colonies were re-inoculated on a blood agar medium (base material made by Merck and Iranian sheep spring defibrinated blood) and incubated at 35°C. After ensuring no growth, they were assigned to Legionella (21, 22). Data were analyzed and reported using Excel 2010 software and descriptive statistics.
3.2. Detection of Legionella
Legionella growth was monitored and recorded on the third, fifth, seventh, 10th, and 14th days. The colonies that appeared on the first and second days were not Legionella (by biochemical tests), and the colonies that appeared on the third and later days were thoroughly examined. Also, 0.5 Ml of sodium-containing sample was stored in sterile Eppendorf tubes for PCR in a -70°C freezer.
Legionella colonies were detected under ultraviolet light by their size, color, type, special, and fluorescence properties. In addition, the grown colonies were re-cultured on BCYE agar, and their appointment to Legionella was confirmed after ensuring that they did not grow. Legionella colonies were white-gray or blue-green, convex, and shiny with 2 - 4 mm diameter. The central part of the young colonies appeared as light gray, granular-like with a glass background, while the peripheral part of the colony was light pink or blue.
3.3. Primer Preparation
To select and design specific primers, some points should be considered. Due to the diversity of primer sequences, the sequence of identified genes in genome databases was collected and analyzed to design the best primer. The primers were compared with the sequences recorded in the Genome Bank (Blast) by a set of analysis programs and sequence comparisons (Tables 1 and 2).
| Primers | Sequences |
|---|---|
| F-mip | AAAGGCATGCAAGACGCTAT |
| R-mip | GTATCCGATTTTCCGGGTTT |
| Amplicon size: 260 bp | |
Sequence of Primers for mip Gene
| Primers | Sequences |
|---|---|
| F-16srRNA | AGGGTTGATAGGTTAAGAGC |
| R-16srRNA | CCAACAGCTAGTTGACATCG |
| Amplicon size: 386 bp | |
Sequence of Primers for mip Gene
3.4. DNA Extraction
DNA extraction was performed using a Korean PROMEGA kit.
3.5. Quantitative Examination of Extracted DNA
In this method, using a spectrophotometer, the light absorption of all samples was read at 260 nm, and using the following equation, the DNA concentration of the sample was obtained: C (g/mL) = 50 d. A260.
3.6. Gel Electrophoresis PCR Product
To view the PCR products, the amplicon was electrophoresed on 1% agarose gel and observed in UV light.
3.7. Sequencing of 16srRNA and mip Gene
To confirm the genes, the PCR products of each of the mip and 16srRNA genes were sent to the Pishgam company for sequencing, and the sequence results were BLAST.
4. Results
4.1. Culture Results and Diagnostic Tests
Suspected colonies on BCYE agar were stained with the Gram technique. Legionella organisms are Gram-negative bacilli. Catalase and oxidase tests were performed.
4.2. Morphology of Legionella
Legionella is found as bacilli in fresh cultures and filamentous in old cultures.
4.3. Sodium Hippurate Hydrolysis Test
If the bacterium is L. pneumophila, it releases the enzyme hypurase into the medium, which breaks down the Hippurate to glycine and benzoic acid. The addition of ferric chloride reagent results in a reaction with benzoic acid and a white precipitate.
4.4. Culture on Blood Agar Medium
In this experiment, the bacterium was isolated from the suspected colonies cultured on B after 48 hours of incubation; if the bacteria did not grow, it is a possible confirmation of Legionella because Legionella is a fastidious bacterium that does not grow in a blood agar medium.
4.5. PCR Results
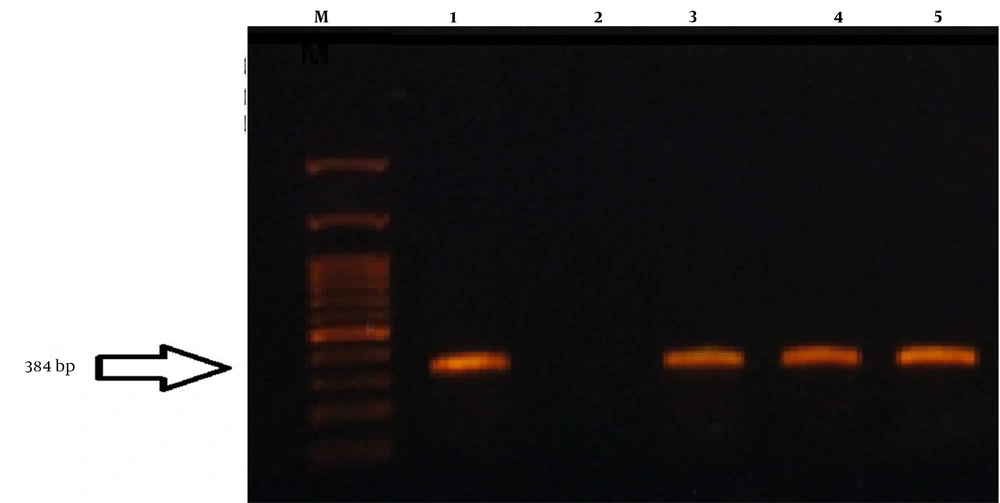
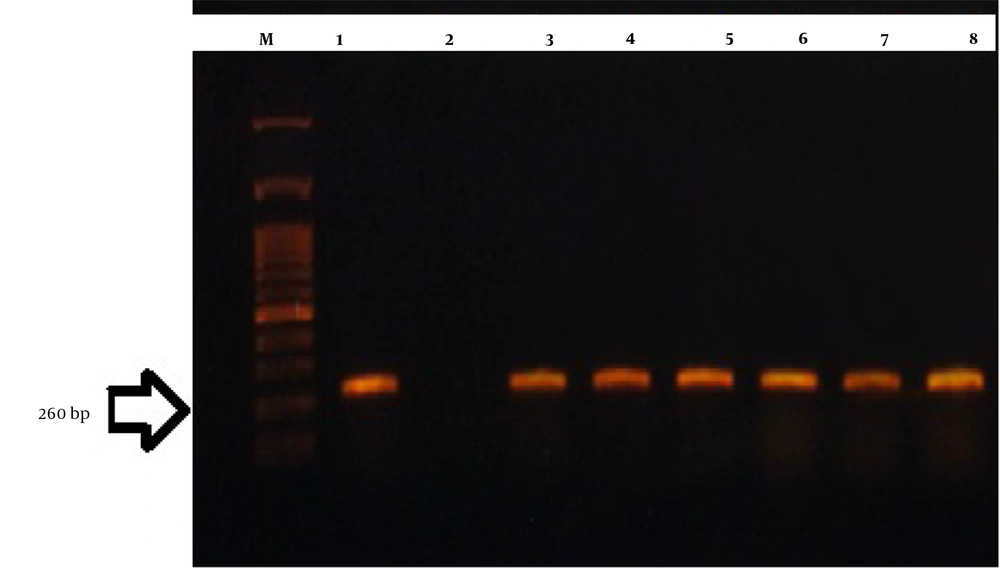
The PCR test to detect the Legionella genus was performed using 16srRNA and mip genes (Figure 1 and 2).
4.6. Mip gene and 16s rRNA Sequence Results
The results showed that mip and 16s rRNA genes had 87% and 70% homology sequences.
4.7. Culture and PCR Results in Water Samples Examined for the Presence of Legionella
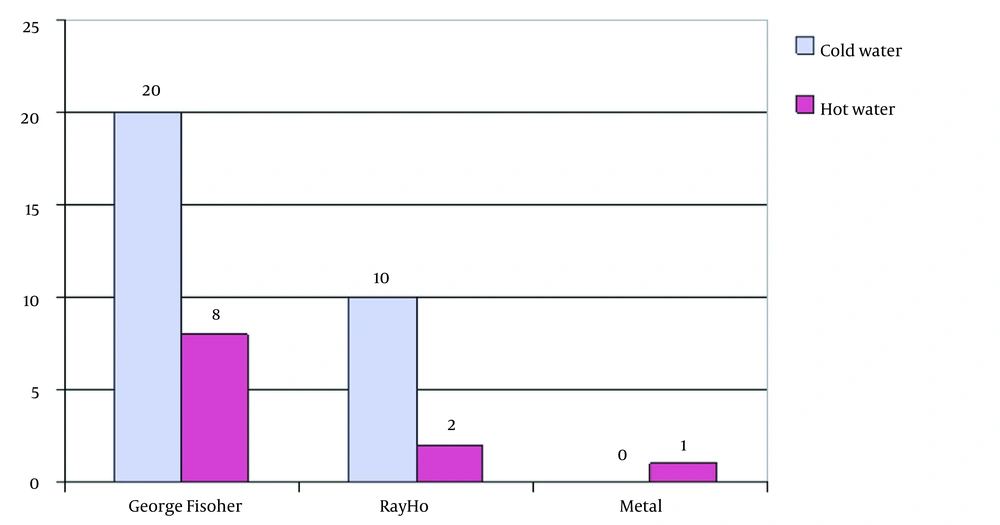
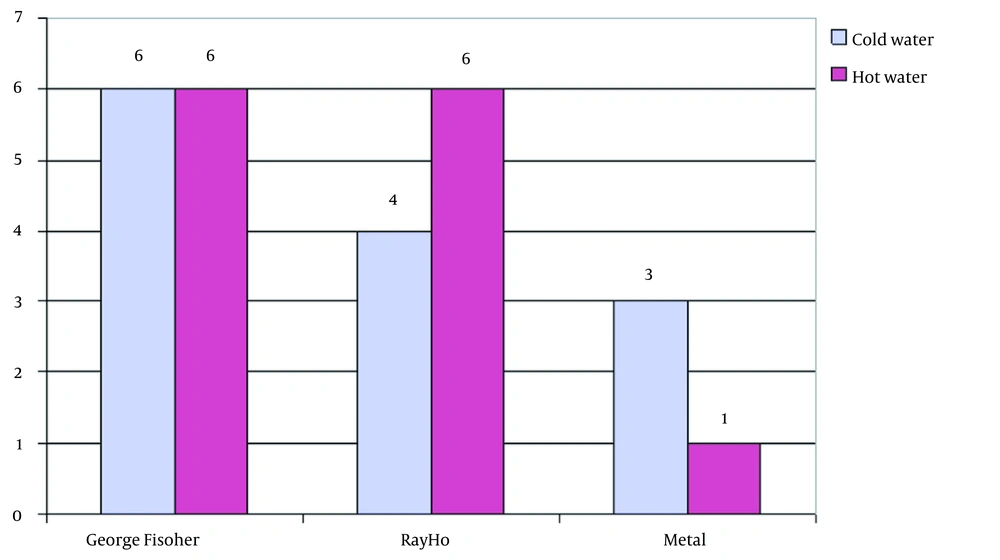
In this study, 61 samples were collected for Legionella pneumophila. Of them, 34 samples were collected from the northern part of the commercial gallery office, in which George Fischer pipes and fittings were used. A total of 22 samples were collected from the northern part, which used Ray Ho pipes and fittings. Five samples were collected from Taleghani hospital, in which metal pipes and fittings were used. The results showed that George Fischer pipes and fittings had very high efficiency and performance in controlling the growth of L. pneumophila.
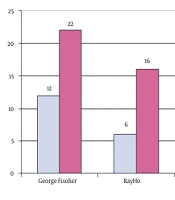
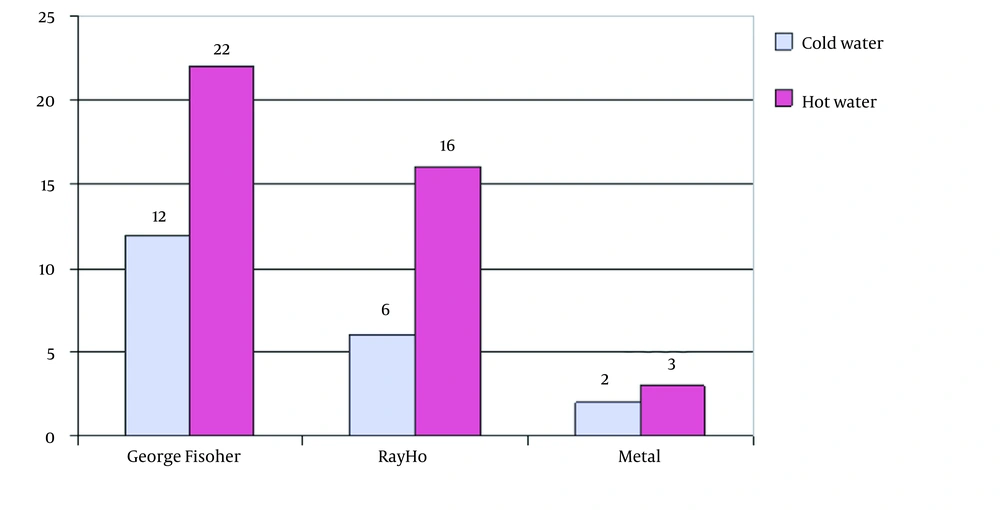
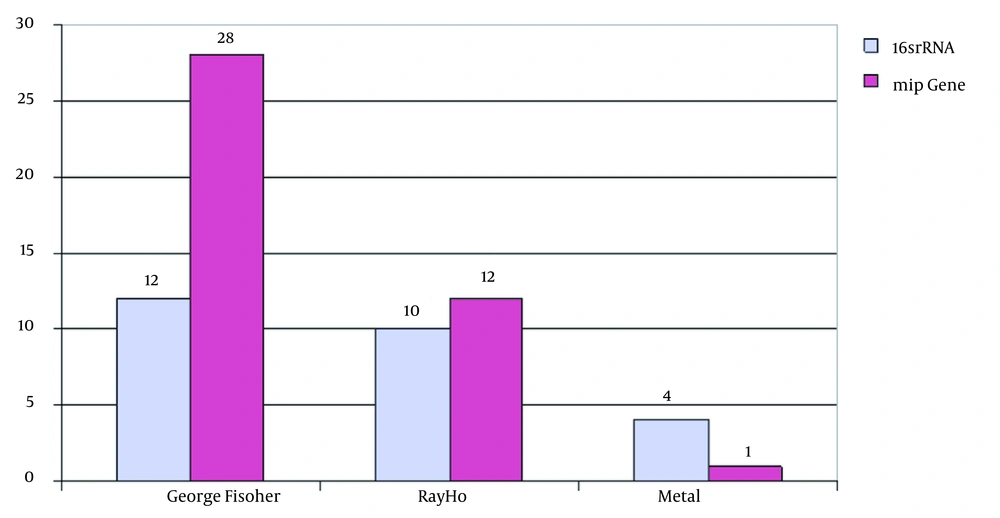
The PCR results showed that out of 61 samples collected, 34 samples were from the George Fischer piping system, 22 from the Ray Ho piping system, and five from the metal pipe of Taleghani hospital. Thus, 35.2% of George Fischer samples, 45.4% of Ray Ho samples, and 80% of Taleghani hospital samples were positive for the 16SrRNA gene. It was also found that 82.3% of George Fischer samples, 54.5% of Ray Ho samples, and 20% of Taleghani hospital samples were positive based on the mip gene (Figure 3-6).
5. Discussion
The vast and increasing range of Legionella diseases and the isolation and identification of species and serogroups of this bacterium from environmental sources and patients indicate the global spread of Legionella species in different countries. Numerous reports of the epidemic and sporadic occurrence of Legionnaires' disease and its related deaths have attracted the attention of researchers and specialists in lung and respiratory diseases, infectious diseases, microbiology, immunology, environment, and genetics (10).
Early diagnosis of legionellosis and epidemiological conditions in the hospital is necessary not only for correct and effective treatment but also for controlling and preventing the subsequent onset of the disease. It seems that due to the high mortality rate of Legionella disease, more effective measures should be taken to prevent the spread of Legionella species from the hospital environment to susceptible patients (7, 10).
Legionella infections in immunocompromised individuals, corticosteroid recipients, organ transplant receivers, the elderly, children, alcohol and drug addicts, smokers, patients with diabetes mellitus, underlying diseases, chronic obstructive pulmonary disease, and intubation pose a serious threat to heart patients more than anyone else. Legionella alone causes a high percentage of nosocomial pneumonia. This bacterium causes 1% to 40% of hospital-acquired pneumonia cases. The mortality rate of legionellosis is 5 - 30%. About 5% of cases of Legionnaires' disease are reported from hospitals (4, 23). Legionella pneumophila was the cause of 3.8% of fatal cases of nosocomial pneumonia. Bacteria of the Legionella genus are isolated from natural water sources, public water reservoirs, water pipes, and even bath showers.
Because various factors are influential in the growth and pathogenicity of Legionella, the factors affecting the growth and survival of bacteria can be primarily identified, and the findings can be used to design an appropriate control method (2, 4, 7). Today, various methods are being studied and performed to control Legionella in hospital and community aquatic environments. Therefore, identifying pathogenic strains in various areas can provide specific ideas for the use of effective and efficient disinfectants to control Legionella.
In a hospital population, there are always patients who are susceptible to infection and are at high risk for Legionella. Water is one of the common sources of Legionella transmission in hospitalized patients. Early diagnosis of legionellosis and epidemiological conditions in the hospital is necessary not only for correct and effective treatment but also to control and prevent the subsequent onset of the disease. It seems that due to the high mortality rate of Legionella disease, more effective measures should be taken to prevent the spread of Legionella species from the hospital environment to susceptible patients (10).
Much research has been done using different methods for removing Legionella from water. In 2005 studied a variety of disinfectants such as ozone, chlorine dioxide, chlorine, monochloramine, copper, and silver to remove amoebae and planktons and used chlorine oxide to remove Legionella (24). Sodium hypochlorite was used to remove Legionella from water, which has a higher oxidation rate than other disinfectants, affecting a wide range of microorganisms, but it has disadvantages such as carcinogenicity and side effects (4). Other methods used to remove Legionella are photocatalytic oxidation (UV at 365 nm) with titanium dioxide. In 2005 used the heat shock method (20).
In 2008 study used the ionization process of copper and silver as a disinfectant to remove L. pneumophila (25). This procedure took four to seven months that was effective in the short term, but the pollution was not eliminated (25). Diamond electrode electrolysis is used to remove and inactivate Legionella (18). In general, the advantages of this research over other research are the long-term bactericidal stability of the George Fischer pipes, no use of chemicals, no carcinogenicity, no corrosion of pipes, no side effects, no need for preparation, and low investment cost.
The results showed that George Fischer pipes and fittings were much less contaminated; out of 34 samples from the George Fischer pipes and fittings system, two samples (samples 20 and 10) showed contamination with Legionella. Thus, the pipe and George Fischer connections showed more than 95% decreases in the growth of L. pneumophila in water, showing the role of this system in controlling infections associated with this bacterium, which causes acute and important problems in the health system, especially in medical centers, hotels, and other administrative, commercial, and recreational centers. Contact with this bacterium and lack of proper treatment can cause the death of infected people.
The results showed that the Ray Ho pipe and fittings system had higher contamination than the George Fischer pipe and fittings system so that out of 22 samples collected from Ray Ho pipe and fittings, 12 samples showed positive Legionella contamination. The results showed that Ray Ho pipes and fittings reduced more than 55% of Legionella contamination, confirming the superiority of the George Fischer pipes and fittings over Ray Ho pipes and fittings.
Examination of Legionella bacterial density in these systems also showed that the George Fischer pipes and fittings are not only less contaminated but also in terms of contamination density than Ray ho pipes and fittings and metal pipes. In the George Fischer pipes and fittings system, only two samples with two bacteria per liter of water were observed, while in Ray Ho and metal pipes, the probability of contamination and bacterial density was high, which indicates the excellent ability of the George Fischer pipes and fittings in controlling Legionella and the inability of these pipes to control Legionella.
The results showed that the average bacterial density in Ray Ho pipe and fittings was more than twice that of the George Fischer pipe and fittings, so Legionella bacterial density in Ray Ho pipe and fittings was in the range of 2 - 16 colonies per liter. In contrast, this density in George Fischer pipe and fittings was only two bacteria per liter, which is even lower than the recommended standards for Legionella density in the piping system of developed countries. A comparison of Legionella density in metal pipes also shows that these pipes are not comparable to George Fischer pipes in terms of both probabilities of contamination and density, so that in these pipes, three out of five samples had contamination. On average, there were more than 43 colonies per liter of water.
The positive results and densities of Legionella bacteria in water samples and their negation in PCR method based on the mip gene indicated the presence of contaminants in water. However, contact with the pipe fittings of George Fischer prevented its growth or caused its destruction. The only evidence of the presence of L. pneumophila DNA in water confirms the effect of George Fischer pipes and fittings in controlling and removing Legionella, which shows that this system is suitable for use in the plumbing of medical centers, hotels, and leisure centers because, in such centers, people are more likely to be exposed to acquired lung pneumonia, which has a high cost of treatment and causes death if not treated properly. The results showed that 28 of the 34 samples of George Fischer pipes and fittings in the culture method were negative but based on a positive mip gene, which indicated VBNC bacterium and L. pneumophila DNA in water, the effect of the tube components on the control of Legionella was proven.
5.1. Conclusions
Legionella stopper pipes and fittings in George Fisher Company have suitable properties for destroying and controlling the growth and density of L. pneumophila in the water supply system of buildings, so it can probably be considered a suitable option for use in piping and water supply systems inside buildings.
5.2. Limitations
One of the limitations of this study was sample collection, financial support, and high research costs.