1. Background
Cirrhosis is a disease, in which the normal liver tissue undergoes fibrosis associated with the formation of degenerative nodules (1). The global prevalence of cirrhosis is unknown; however, it is estimated to be between 0.15% and 0.27% in the United States (2). The risk factors of liver cirrhosis include alcohol abuse, hepatitis B and C virus infection (especially in developing countries), non-alcoholic steatohepatitis (NASH), medications and toxins, congenital diseases, such as Wilson’s disease, alpha-one antitrypsin deficiency, and Budd-Chiari syndrome, autoimmune hepatitis caused by diseases, such as primary biliary cholangitis, primary sclerotic cholangitis, and hemochromatosis (3-5).
One of the major complications of liver cirrhosis seen in nearly one-third of patients is hepatic encephalopathy (HE). Ammonium accumulation is a major cause of HE and a strong predictor of its severity in patients with chronic liver failure. In patients with liver cirrhosis, the synergy between hyperammonemia and inflammation is associated with impaired cognitive function, memory dysfunction, and learning problems (6). The factors predisposing to encephalopathy include a history of gastrointestinal bleeding, use of diuretics, infections, hyponatremia, surgery, constipation, renal failure, anaemia, and hypoglycaemia (7, 8).
Magnesium is a mineral involved in bone health, energy production, metabolic processes, enzymatic activities, protein synthesis, fat production, nucleic acid replication, transport systems, muscle contraction, and immune function (9, 10). The signs and symptoms of magnesium deficiency include tremors, muscle spasms, personality change, gastrointestinal problems, and altered bone metabolism. Magnesium deficiency is linked with renal disease, use of diuretics, gastrointestinal diseases (particularly malabsorption), thyroid and parathyroid disorders, and postsurgical stress (10).
2. Objectives
Magnesium imbalance has been reported in patients with liver diseases, such as liver cirrhosis and fatty liver (11-13). However, there is insufficient data to draw a definite relationship between magnesium deficiency and liver diseases or its complications. Hence, in the present study, we assessed the association between serum magnesium concentration and HE in patients with liver cirrhosis.
3. Methods
This study was conducted in the internal disease ward of Shariati Hospital of Isfahan, Iran, from August 2018 to December 2019 (registration number: 5033). We included all patients diagnosed with liver cirrhosis secondary to any cause. The diagnosis was made by a gastroenterologist based on clinical, biochemical, and histopathological findings. The patients who had a history of cardiac/cerebral stroke, using sedatives, concussion, transjugular intrahepatic portosystemic shunt, or shock, and those with fever were excluded from the study.
Hepatic encephalopathy was diagnosed based on clinical examination and biochemical tests. Finally, 90 cirrhotic patients were included in the study and divided into two groups: with or without HE. The study was approved by the Ethics Committee of the Najafabad Islamic Azad University (IR.AIU.NAJAFABAD.REC.1397.043). Also, the guidelines of the Declaration of Helsinki were followed, and written informed consent was obtained from all participants.
Patients’ sex and age, cause of cirrhosis, model end-stage liver disease (MELD) score, and Child-Pugh-Turcotte (CTP) class (A, B, or C) were recorded (14). Hypomagnesemia was defined as a serum magnesium concentration of less than 1.7 mEq/L (15). Serum magnesium was measured using a commercial kit via photometry and the Xylidyl blue chromogenic assay in an alkaline solution (Pars Azmoon, Iran).
Data analysis was conducted in Statistical Software Package for Social Science (SPSS) (2015 IBM SPSS for Windows, v. 24.0). Categorical data and continuous data were presented as percentages and mean ± standard deviation (SD), respectively. The chi-square test and student t-test were used to compare categorical and continuous data between the study groups, respectively. Nonparametric tests were used if the data were not normally distributed. A P-value of less than 0.05 was considered statistically significant.
4. Results
Table 1 shows the participants' demographic characteristics. Of the 90 cirrhotic patients included in this study, 43 (47.8%) were female, and 47 (52.2%) were male. The average age was 64.1 ± 13.7 years. The most common causes of cirrhosis were as follows: cryptogenic (71.1%), hepatitis B (7.8%), hepatitis C (8.9%), primary sclerosing cholangitis (PSC) (2.2%), hemosiderosis (4.4%), and other reasons (5.6%). The average MELD score was 15.0 ± 5.6. Regarding CTP, 38.9% of the participants were in class A, 41.1% in class B, and 20.0% in class C.
| Characteristics | Values |
|---|---|
| Age (y) | 64.1 ± 13.7 |
| Sex | |
| Male | 47 (52.2) |
| Female | 43 (47.8) |
| Cirrhosis causes | |
| Cryptogenic | 64 (71.1) |
| HBV | 7 (7.8) |
| HCV | 8 (8.9) |
| PSC | 2 (2.2) |
| Hemosiderosis | 4 (4.4) |
| Others | 5 (5.6) |
| MELD score | 15.0 ± 5.6 |
| Child-Pugh | |
| A | 35 (38.9) |
| B | 37 (41.1) |
| C | 18(20.0) |
| Serum magnesim (mEq/L) | 1.84 ± 0.3 |
| Hypomagnesaemia | 31 (34.4) |
Demographic Characteristics (N = 90)
Mean serum magnesium concentration was 1.84 ± 0.3 mEq/L, and 31 patients (34.4%) had hypomagnesaemia. Table 2 demonstrates the patients’ biochemical parameters based on serum magnesium status. Age and sex had no significant effects on serum magnesium concentration (P-value = 0.134 and 0.212, respectively). The distribution of cirrhosis etiologies was significantly different between patients with or without hypomagnesemia (P-value= 0.005). The most frequent diagnoses among those with hypomagnesemia were cryptogenic cirrhosis (n = 19, 61.2%), cirrhosis due to hepatitis C virus infection (n = 4, 12.9%), PSC (n = 2, 6.5%), hemosiderosis (n = 4, 12.9%), and other reasons (n = 2, 6.5%). There was a significant difference in the distribution of Child-Pugh class among patients with or without hypomagnesaemia, and most patients with hypomagnesemia were in classes B and C (P-value = 0.027). The means of the MELD score were 16.5 ± 5.8 and 14.3 ± 5.4 in those with hypomagnesaemia and without hypomagnesaemia, respectively (P-value = 0.08).
| Variables | With Hypomagnesaemia (N = 31) | Without Hypomagnesaemia (N = 59) | P-Value |
|---|---|---|---|
| Encephalopathy | 25 (80.6) | 20 (33.9) | < 0.001 |
| Age (years) | 61.1 ± 16.7 | 65.7 ± 11.8 | 0.134 |
| Sex | 0.212 | ||
| Male | 19 (61.3) | 28 (47.5) | |
| Female | 12 (38.7) | 31 (52.5) | |
| Cirrhosis causes | 0.005 | ||
| Cryptogenic | 19 (61.2) | 45 (76.3) | |
| HBV | 0 (0) | 7 (11.9) | |
| HCV | 4 (12.9) | 4 (6.8) | |
| PSC | 2 (6.5) | 0 (0) | |
| Hemosiderosis | 4 (12.9) | 0 (0) | |
| Others | 2 (6.5) | 3 (5.0) | |
| MELD score | 16.5 ± 5.8 | 14.3 ± 5.4 | 0.08 |
| Child-Pugh | 0.027 | ||
| A | 9 (29.0) | 26 (44.1) | |
| B | 11 (35.5) | 26 (44.1) | |
| C | 11 (35.5) | 7 (11.9) |
Biochemical Variables in the Groups
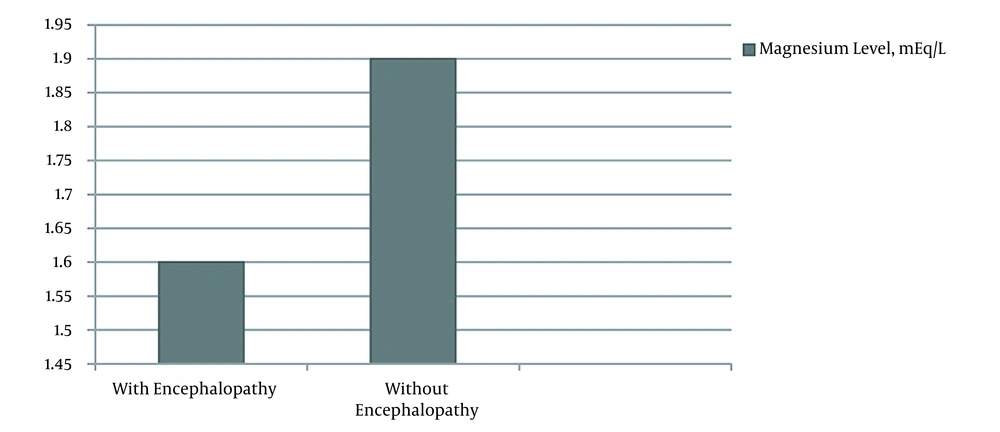
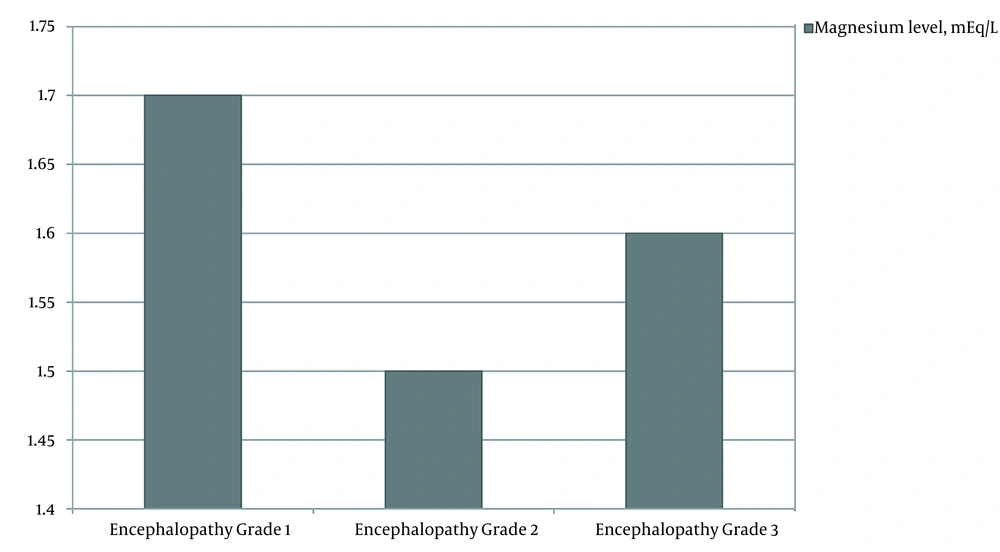
Hepatic encephalopathy was diagnosed in 45 (50.0%) of the patients 25 (80.6%) of those with hypomagnesemia and 20 (33.9%) of patients without hypomagnesaemia). The mean level of serum magnesium in patients with encephalopathy was 1.6 ± 0.2 mEq/L in comparison with 1.9 ± 0.2 mEq/L in those without encephalopathy (P-value < 0.001, Figure 1). There was no significant relationship between encephalopathy grade and serum magnesium concentration (P-value = 0.377, Figure 2).
5. Discussion
In the present study, we evaluated the link between plasma magnesium concentration and HE in patients with liver cirrhosis. Hypomagnesemia was seen in nearly one-third of cirrhotic patients, most of whom were in Child-Pugh classes B and C. The prevalence of hypomagnesemia was higher among cirrhotic patients with HE than those without this complication. Age, gender, MELD score, and encephalopathy grade were not significantly associated with hypomagnesaemia.
Patients with liver diseases have been reported to have reduced serum magnesium concentration (12, 16-19). Gowda and Tembad reported hypomagnesemia among patients with liver cirrhosis and individuals with alcohol-induced liver disease (16). Hypomagnesemia has also been reported in patients with alcoholic and non-alcoholic fatty liver (19). Saxena et al. observed significantly lower serum magnesium levels in patients with liver cirrhosis compared to healthy controls (18). Magnesium depletion was also reported to be a serious problem in those with chronic terminal liver cirrhosis (17). A study noted significant cellular magnesium depletion in patients with cirrhosis (20). Some studies have also published conflicting results. In one study, no significant difference was observed comparing serum magnesium levels between patients with cirrhosis and healthy individuals (21). As an explanation, it has been reported that factors, such as alcohol intake, secondary hyperaldosteronism, use of diuretics, and hypoalbuminemia can affect serum magnesium concentration (22). Other possible causes that can decrease serum magnesium levels include inadequate dietary magnesium absorption, gastrointestinal loss, alcohol intake, hypophosphatemia, diuretic therapy, and respiratory alkalosis (23). The findings of the Third National Health and Nutrition Examination Survey Cohort (NHANES) suggested that magnesium supplementation was associated with a lower mortality rate due to liver diseases, particularly alcoholic liver disease and liver steatosis (24).
We observed a higher prevalence of hypomagnesemia in patients with HE. Therefore, it can be concluded that hypomagnesemia may be a risk factor for encephalopathy in cirrhotic patients. Accordingly, Lopes et al. demonstrated that lower serum magnesium levels were related to a higher risk of HE among both donors and recipients of liver grafts during the immediate postoperative period (25). A study on rats suggested that magnesium administration might help reduce HE complications, such as cognitive and locomotor dysfunction (26).
In our study, most cirrhotic patients with hypomagnesemia fell into the Child-Pugh classes of B and C. In agreement with our findings, Nangliya et al. indicated a significant decrease in serum magnesium levels with liver cirrhosis progression (12). Hypomagnesemia is associated with lower hepatic magnesium levels, increasing collagen deposition in hepatocytes, and aggravating cirrhosis (27). Magnesium deficiency is also associated with cardiometabolic, neurologic, and muscular complications, and its proper and timely treatment is necessary to prevent the development of HE in patients with liver cirrhosis (28).
The present study has some limitations. We did not investigate the potential causes of magnesium deficiency (inadequate dietary intake, excessive excretion, etc.). In addition, the small sample size limits the generalizability of our results.
5.1. Conclusions
The prevalence of hypomagnesaemia was relatively high in patients with liver cirrhosis complicated with HE. Hypomagnesaemia was associated with the severity of liver cirrhosis based on the Child-Pugh score. Magnesium supplementation in cirrhotic patients may be helpful in preventing disease progression; however, further studies, and particularly clinical trials, should be conducted in this regard.