1. Background
Stroke is one of the most common and debilitating neurological lesions and the second most common cause of death worldwide (1). In general, defects after brain injury are mainly in three parts: physical, cognitive, and emotional-behavioral (2). According to available reports, cognitive injuries are widespread in people with stroke, and many complain of these defects (3). The outbreak of cognitive disorders has been reported up to 96% three months after stroke, which can significantly affect patients' function in their daily activities (4).
Post-stroke cognitive impairment includes disorders in executive function, memory, attention, language, and spatial visual function (5). Working memory is an impaired executive function in stroke patients (6). Working memory is the ability to keep information active to guide purposeful behavior and includes temporary data, momentary manipulation, and information control (7). Defecting working memory in the chronic stage of stroke is still prominent and may affect other executive functions and the formation and retrieval of episodic memory. Working memory mediates the access between short-term and long-term memory (8). Therefore, finding ways to reduce these problems and accelerate the process of cognitive rehabilitation can improve the quality of life of these people.
Cognitive rehabilitation includes various methods that various rehabilitation professionals can perform to improve cognitive functions, and its purpose is to optimize individuals' functions in their activities (9). However, a rehabilitation procedure, which is a long-term period, should be easy and exciting to maintain the active participation of the patient, and computer-based cognitive interventions appear to have this feature (10). According to studies, cognitive defects and problems caused by brain injuries can be improved by appropriate computer-based programs in people suffering from stroke (11-13).
Another therapeutic approach to the cognitive problems of stroke patients is using repetitive Transcranial Magnetic Stimulation (rTMS) to improve their working memory. The rTMS stimulations include low-frequency (below 1 Hz) or high-frequency (1 to 50 Hz) stimulations using repetitive pulses (14). When rTMS waves are repeatedly applied, they can modulate the stimulability of the cerebral cortex by increasing or decreasing it depending on the type of stimulation (15). In addition, when rTMS is repetitive, long-term changes occur in cortical function and can be used as cognitive enhancers in healthy and disease conditions (16). Nevertheless, studies examining the effect of rTMS on executive functions and working memory of stroke patients are limited and varied (17, 18), and based on a recent systematic review and meta-analysis (2021), not enough evidence is available yet (19).
On the other hand, although minor side effects have been reported for rTMS (20), it is necessary to select the appropriate stimulation parameters such as stimulation duration, pulse number, and stimulation location to establish the routine use of this method after stroke. A systematic review stated that rTMS positively influences the improvement of cognitive ability in stroke people; however, the evidence is still limited. The authors of the review study concluded that one of the limitations of their study was heterogeneity among studies due to different evaluation scales and stimulation parameters such as stimulation position (Left DLPFC/Right DLPFC/Bilateral Frontal Lobe), intensity (60 - 120% MT), and frequency (0.5 – 10 Hz). So, more investigations are required to explore the optimum stimulus outcomes (21). In this regard, we aimed to deal with the magnitude of stimuli in each session using high-frequency rTMS. To our knowledge, similar studies used high-frequency stimulation with a minimum pulse of 700 (22) and a maximum pulse of 2000 for 20 minutes (5, 23). Nevertheless, it is unclear whether lower doses or fewer sessions can also be effective.
Therefore, the question is whether applying rTMS at a lower dose than in a previous study (5) can improve work memory.
2. Objectives
The present study aimed to investigate the combined effect of rTMS with 600 pulses for six minutes in 15 sessions and computer-based cognitive interventions.
3. Methods
3.1. Participants
In this double-blind, randomized clinical trial, 18 stroke patients (hemorrhagic or ischemic) diagnosed by a neurologist were selected by convenience sampling from those referred to the Qasr Comprehensive Rehabilitation Center in Isfahan. Inclination criteria were having a stroke for the first time based on available medical records, age between 55 and 75 years, passing at least six months and up to two years after stroke, lesion on the left side of the brain, predominance on the right side of the body evaluated by Edinburgh Handedness Inventory, no obvious symptoms of psychosis in a person according to the doctor's diagnosis, no history of seizure in the last six months, no use of drugs affecting cognition, not having unilateral neglect by Catherine Bergego Scale, having a minimum literacy, not receiving rTMS services before the enrollment, and obtaining a minimum score (8 ± 2) on the Digit Span subscale of the Wechsler Adult Intelligence Scale (WAIS). This number (8 ± 2) is selected based on the average age group of 55 years and above, which is reported in the Persian version of the Wechsler memory test by Nasiri and Bagheri Yazdi (24). Exclusion criteria included the absence from more than two sessions, stroke recurrence, and seizure during the study period. The sample size using a previous study with 80% power and 0.05 alpha (25) was determined to be nine people in each group. The Ethics Committee of Shahid Beheshti University of Medical Sciences approved this study with the code IR.SBMU.RETECH.REC.1399.277. It was registered as a clinical trial with the code IRCT20201016049041N1.
Due to the consecutive sampling method in the present study, random allocation was also conducted consecutively. It is noteworthy that only one rehabilitation center was sampled. This study utilized a simple randomization procedure, and the randomization unit was individual. For this aim, individuals with even numbers (by order of referral) were included in the experimental group, and participants with odd numbers were included in the control group.
3.2. Tool
N-back task: This test is a cognitive function assessment task related to executive functions. Because this task involves maintaining and manipulating cognitive information, it assesses the working memory (26). This test has two components, visual and auditory, and the visual type was used for the present study. In the visual type, several visual stimuli with a distance of 1,800 milliseconds appear as series on the screen, and the person must compare each stimulus with the previous one. If a stimulus is similar to the previous one, the person presses the number one key, and if it is not similar, he/she presses the number two key on the keyboard. Two scores were obtained from this test: incorrect recognition percentage with a reliability coefficient of 0.51 and non-recognition percentage with a reliability coefficient of 0.76 (27). In the present study, the accuracy of the N-back task was reported.
3.3. Procedure
Before the study, stroke patients received routine treatments, including rehabilitation services for physical-motor problems, such as two sessions of physiotherapy and two sessions of occupational therapy per week. First, all subjects were initially assessed on the working memory subscale through the WAIS to enter the study. Before the interventions, the N-back test measured the working memory of all subjects. Then, individuals were randomly divided into experimental and control groups with an equal number of genders. Given that the present study was a double-blind clinical trial, the researcher was unaware of which group was experiment or control from the beginning of the evaluation and intervention process. Also, the subjects did not know which of these two groups they belonged to.
After the initial evaluation, all individuals participated in rTMS intervention sessions. Because the prefrontal cortex and especially the dorsolateral region are involved in working memory (13), in this study, the site of stimulation was on the left Dorsolateral Prefrontal Cortex (DLPFC), determined by the Beam F3 system (28). The coil was placed tangentially to the scalp, and its handle was 45 degrees to the back and away from the midline (29). In this study, a high-frequency repetitive rTMS protocol was used at the stimulation of 10 Hz, known to create facilitation effects in the motor cortex (30, 31). During each session, frequently activated rTMS (Super Rapid 2, Magstim, England) with a frequency of 10 Hz included 60 one-second stimulations (10 pulses) with a five-second rest interval between stimulations and a total of 600 pulses with 100% power of motion threshold, which was the lowest rTMS stimulation applied in the left motor cortex defined in 10 consecutive stimulations of visible contraction of the right thumb (25). The device operator conducted the rTMS intervention sessions who was aware of group allocation. The rTMS intervention was done in 15 sessions of six minutes for each patient three days a week. The rTMS of the control group had similar conditions to the active rTMS; the exception was that changing the coil angle prevented the waves from reaching the brain, and the coil was at a 45-degree angle to the surface of the skull. This condition gave the person a similar somatosensory sensation of rTMS, except that it did not affect the brain (25).
In addition to rTMS intervention, both groups received computer-based cognitive therapy services using Captain's Log software in 15 sessions of 30 to 40 minutes three days a week (32, 33). This treatment was performed for individuals immediately after the rTMS by an occupational therapist. The practices used were related to Captain's Log software's working memory rehabilitation practices. These practices have three levels for three age groups, children, adolescents, and adults. The treatment plan for this study was based on the adult level. Each level has 15 stages, each of which takes an average of 1.5 to 3 minutes, increasing the difficulty level of the practices as the stages increase. For example, stage 2 practices have more challenges than stage 1 practices. In all practices, as the level of practice increases, the number of images increases, and distraction factors such as extra sound and additional images are also presented during the practice.
Practices were as follows.
(1) Patterns' sequence practice: First, two lights such as red and blue (or two images) are lit in a random order, and after a few seconds, references should turn on the lights (or images) according to the order performed. In the higher stages, the light number and lightning times increase, and the images change to different objects.
(2) Puzzle power: A table of nine cells containing three categories of images with three colors is displayed at the beginning. Then, some cells are emptied, and the references have to put the images extracted from the table in their previous correct place. In higher stages, the number of images and their variety increase.
(3) Matching game: First, two images are visually shown (in writing) to the references, and they are asked to memorize these two and then find them among other images. The number of images increases as the level of practice increases.
(4) Where is my car? First, we have an eight-cell table. Initially, a single-color car is displayed in a specific sequence inside three cells. The displayed machines are then removed from the table, and the references must replace the machines displayed in the same order. The number of images increases as the level of practice increases.
(5) What color did I forget? First, two colors are shown. Then, the software removes one of the colors, shows only one color, and asks the references which color has been removed. The practice is repeated several times, and in the more difficult stage, four images of a dress with four different colors are demonstrated. Then, three images of the same dress with the same colors are displayed (one of the colors is removed). The person must choose the forgotten color from the options.
Practices for participants are started from level 1 (lowest level of difficulty) in all five practices. Next, depending on the number of correct answers or the response speed, the software automatically increases the complexity level of the practices, or in the case of incorrect answers or slow responses, the complexity of practices is reduced. At the end of each session, information about the process of performing the practices for the person is stored in the software, and in the subsequent sessions, the training levels continue for that person.
At the end of the therapy sessions of both groups, working memory in the posttest was re-evaluated by the N-back test.
3.4. Statistical Analysis
Data were analyzed using SPSS 21 software at a significance level of P < 0.05. The independent t-test and chi-square test were used to evaluate the differences in demographic characteristics between the two groups. Also, the level of pre-study work memory status (Wechsler test) between the two groups was assessed by the independent t-test. Shapiro-Wilk test was utilized to measure the normality of the data; then, the paired t-test for within-group comparisons and the independent t-test for between-group comparisons in posttest were applied.
4. Results
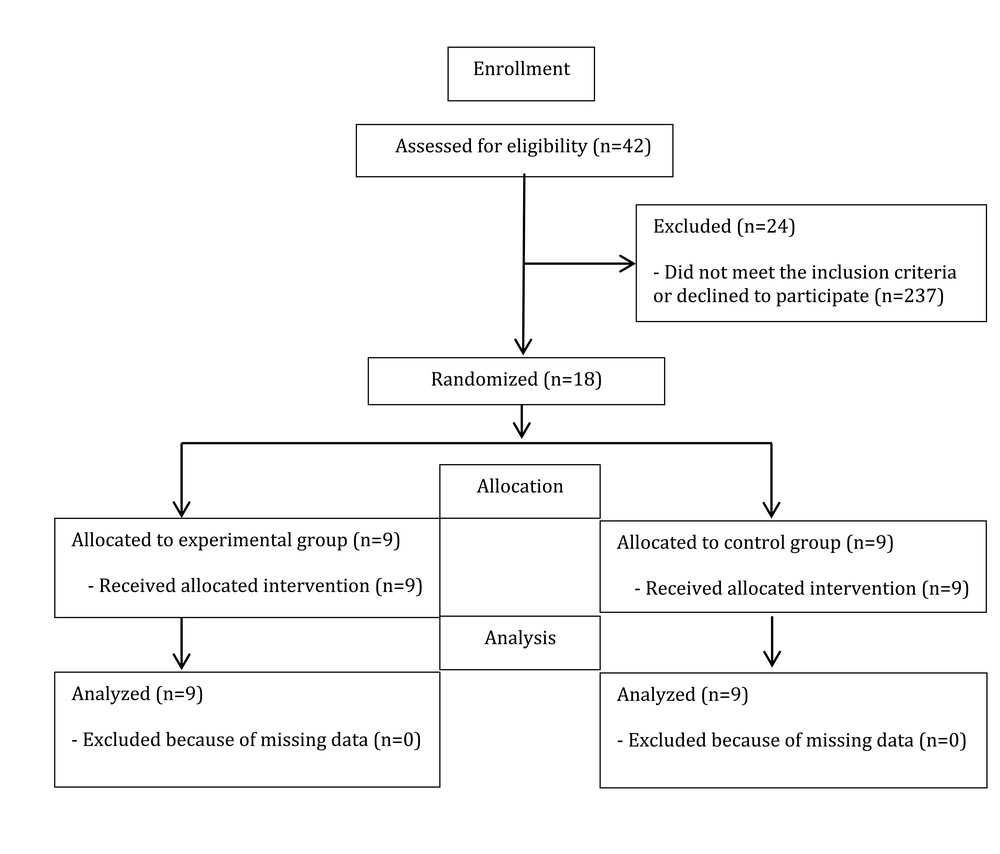
In this study, 18 stroke patients (nine in each group) completed the study protocol and were analyzed (Figure 1). The normality results revealed a normal distribution for the working memory variable. There was no significant difference between the two groups in demographic characteristics, including age, level of education, and duration after stroke (Table 1, P > 0.05). In addition, there was no significant difference between the two groups in terms of working memory based on the WAIS test and the previous N-back subscale, indicating homogeneity between the two groups before the interventions (Table 2).
| Variables | Intervention Group a | Control Group a | P-Value |
|---|---|---|---|
| Wechsler test score | 7.67 ± 1.50 | 7.33 ± 1.50 | 0.956 |
| Duration after stroke (mon) | 13.33 ± 4.97 | 12.11 ± 4.28 | 0.584 |
| Age (y) | 66.22 ± 4.08 | 62.56 ± 4.00 | 0.494 |
| Sex | 1.000 | ||
| Male | 5 (55.6) | 5 (55.6) | |
| Female | 4 (44.4) | 4 (44.4) | |
| Education | 0.167 | ||
| Middle school | 0 (0) | 2 (22.2) | |
| Associate degree | 2 (22.2) | 3 (33.3) | |
| Bachelor’s degree | 4 (44.4) | 2 (22.2) | |
| Bachelor of science | 3 (33.3) | 2 (22.2) |
a Values are expressed as mean ± SD or No. (%).
| Variables | Pretest | Posttest | Differences | Within-group Comparison (Pretest and Post-test) | |||||
|---|---|---|---|---|---|---|---|---|---|
| P-Value | 95% Confidence Interval | t | |||||||
| Lower | Upper | ||||||||
| Work memory (score) | |||||||||
| Experimental group (n = 9) | 57.78 ± 8.090 | 74.67 ± 9.138 | 16.8 ± 5.278 | < 0.001** | -20.94 | -12.83 | 9.599 | ||
| Control group (n = 9) | 58.22 ± 6.760 | 65.67 ± 6.305 | 7.44 ± 1.424 | < 0.001** | -8.53 | -6.35 | 15.684 | ||
| Between-group comparison | |||||||||
| P-value | 0.901 | 0.027* | < 0.001** | ||||||
| Lower | Upper | Lower | Upper | ||||||
| 95% Confidence interval | -7.913 | 7.024 | 1.074 | 16.926 | |||||
| F-value | 0.642 | 0.404 | |||||||
a * P < 0.05, ** P < 0.001
Data analysis after the interventions showed that the use of rTMS in the left DLPFC region along with the cognitive rehabilitation in the experimental group (mean posttest: 74.67) significantly improved the working memory of stroke patients compared to those who received just the cognitive rehabilitation in the control group (mean posttest: 65.67) (P = 0.027). Also, the rate of change in the N-back score within-group comparison was significant for both groups. In fact, both groups scored higher in the posttest, but the rate of change was greater in the experimental group (mean difference: 16.8) than in the control group (mean difference: 7.44) (Table 2).
5. Discussion
Working memory impairment is evident even in the chronic stage of stroke, caused by lesions in a widespread frontoparietal network (8). Although the patients in the present study were in the chronic phase, the research results showed that people who used rTMS in combination with computer-based cognitive rehabilitation had a better function in their working memory compared to cognitive rehabilitation alone. In some previous studies, the use of rTMS improved cognitive function and quality of life in patients with cognitive lesions following stroke, which was consistent with the results of the present study (5, 22). However, in Kim's study, a significant effect of rTMS on the working memory of stroke patients was not observed, which could be due to the participants' characteristics such as age and the number of intervention sessions (18). The rTMS intervention sessions were longer in the present study than in Kim's. Therefore, long-term cognitive improvement seems to be related to the number of stimulation sessions/days, so that more stimulation sessions could lead to long-term positive effects (19). Guse et al. also reported that high-frequency rTMS (10 - 20 Hz) probably resulted in a significant cognitive improvement when applied to the left DLPFC within the range of 10 - 15 consecutive sessions (34).
Excessive use of DLPFC as a stimulant target may be attributed to its known role in working memory and its safety and feasibility for rTMS. However, DLPFC is a large region of the association cortex with several distinct functional networks, and the territory of these networks varies between individuals. So, the cognitive benefits of rTMS interventions could vary between individuals as a function of the stimulated networks (35). Li et al. suggested that rTMS-induced neuroplasticity both in the stimulated regions and other regions related to the functional network using resting-state functional magnetic resonance imaging (23).
Although the patients in the present study were in the chronic phase, the combined use of rTMS and cognitive interventions significantly affected patients' working memory. The rTMS may lead to changes in endogenous neurotransmitters (GABA and glutamate) and neuromodulators (DA, NE, 5-HT, and ACh) that are essential in regulating neural activity in the cerebral cortex. It seems that according to the theory, the induce of long-term potentiation (LTP) and long-term depression (LTD) also may affect the plasticity of nerve cells in the brain (36). Nevertheless, this effect should be interpreted with caution.
The rTMS intervention in this study included 60 stimulations with a frequency of 10 Hz for a total of 600 pulses with a power of 100% of the motor threshold for six minutes, which was lower than in similar studies (5, 22, 23). Therefore, this protocol can effectively improve working memory when combined with computer-based cognitive rehabilitation interventions. Given the cost of rTMS interventions and their potential risks, it is imperative to use effective protocols that are also cost-effective.
In the within-group comparison, the rate of working memory changes before and after the intervention was significant in both experimental and control groups; in fact, both groups obtained higher scores on working memory assessment in the posttest. Therefore, cognitive rehabilitation alone could improve working memory. Compared to conventional paper/pencil cognitive rehabilitation, computer-based cognitive rehabilitation has the flexibility to adjust cognitive education based on each patient's specific neuropsychological patterns so that the affected area can be better stimulated and shortens the treatment time by providing immediate feedback. Stroke patients may also be more motivated to seek treatment (37). However, since the changes in working memory were greater in the experimental group than in the control group, it seems that rTMS with cognitive rehabilitation may provide a positive synergistic effect due to the goal of improving the neuroplasticity in stroke rehabilitation (20), thereby accelerating the process of working memory recovery.
However, in order to make better use of rTMS in clinical practice, it must be considered that the location of the brain injury varies from patient to patient. Therefore, the findings may differ based on brain activity changes using neuroimaging with neuropsychological tests. The rTMS affects not only the cerebral cortex below the stimulation site but also the areas of the brain associated with neural network-based function (19). Therefore, one of the limitations of this study was that the brain circuits affecting working memory were not monitored using diffusion tensor imaging (DTI) or functional magnetic resonance imaging (FMRI). Also, the persistence of rTMS effects on working memory was not investigated in this study, so more research is needed in this field.
5.1. Conclusions
Although cognitive rehabilitation using software is effective on the working memory, its combination with rTMS in the left DLPFC region could have a greater effect on improving the working memory in people with chronic stroke.