1. Background
Hypertensive disease, which is a condition with increased risk for subsequent adverse events, including atherosclerotic cardiovascular disease, heart failure, microvascular complications, and type 2 diabetes mellitus (T2DM), refers to a lasting increase in blood pressure (BP) with heterogeneous genetic and environmental factors (1, 2). Several epidemiological studies have indicated that hypertension is an insulin-resistant state. On the other hand, according to the acute effects of insulin on sympathetic nervous system (SNS) activity, the transmission of membrane transport cations, renal sodium reabsorption, cell proliferation, and lipid metabolism, decreased insulin sensitivity is possibly associated with hypertension (1, 3). Hypertension is well known as oxidative stress disease. The reactive oxygen species (ROS) production is increased in multiple organs of almost all experimental models of hypertension (4).
Regulation of oxidative stress is one of the major pathophysiological mechanism and risk factors for metabolic disorders, including diabetes and hypertension (2, 4). The thioredoxin (TRX) system plays an important role in redox signaling antioxidant defense and functions as a protection against oxidative stress (5). Recent studies have shown that thioredoxin interacting protein (TXNIP), which is an important TRX binding protein, has a reciprocal function with TRX in the pathogenesis of diseases, such as diabetes (5, 6). TXNIP plays a key role in activating molecular signals, such as vascular function and glucose and lipid metabolism, due to its interaction with the thioredoxin system. Studies have identified that hyperglycemia and hyperlipidemia stimulate TXNIP (5, 6). On the other hand, insulin inhibits TXNIP production, and this negative feedback limits TXNIP stimulation by chronic hyperglycemia. One possible important compensatory mechanism to protect beta cells from oxidative damage and apoptosis is the suppression of TXNIP by insulin (7). Research shows that thiol oxidoreductase thioredoxin and TXNIP play an important role in adipose tissue physiology, carbohydrate metabolism, insulin sensitivity, inflammation, and blood pressure regulation. Therefore, changes in the thioredoxin system and TXNIP could be considered a new therapeutic target for blood pressure control and other metabolic disorders (5).
It has been suggested that non-pharmacological interventions, such as exercise training and physical activity, effectively control and reduce blood pressure. Regular physical activity improves lipid and glucose metabolism by increasing high-density lipoprotein cholesterol (HDL-C) and insulin sensitivity and reducing low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) (8). Exercise training by increasing TXN levels and decreasing TXNIP in red blood cells and skeletal muscles enhances the ability of the antioxidant defense system and eliminates ROS (9). These findings suggest that regular exercise reduces the risk of chronic diseases and improves physical capacity through antioxidant protection, redox regulation, and improving metabolic state (10). Regular aerobic exercise training improves glycemic control and reduces body weight and risk of cardiovascular disease (8). Resistance exercise training is also suggested by increasing nitric oxide (NO) metabolites as an effective factor in reducing systolic and diastolic blood pressure (SBP and DBP, respectively) (11). On the other hand, stretching exercise training can decrease SBP and DBP by increasing flexibility and decreasing central and peripheral arterial stiffness (12). Therefore, it seems that combining these three types of exercise training can improve and adjust the risk factors for cardiovascular disease. Despite the importance of attention to physical activity and exercise training, environmental and social barriers, such as lack of available resources and facilities, commuting problems and costs, daily occupations and lack of time, and lack of awareness of the benefits of physical activity make it difficult to start a regular exercise program (13). One of the ways to overcome these obstacles is to perform the telemonitoring exercise. Today, with technological advances, such as smartphones, high-speed internet, and mass communication software, exercise training can be done at home with the remote supervision of experienced and trained professionals as complementary medical therapy. Hence, telemonitoring exercises, such as home-based training (HBT) programs can emerge as alternatives for increasing compliance with gym-based training (GBT) programs. Doing HBT with telemonitoring guidance improves the quality of life, the feeling of empowerment in changing the lifestyle, and self-efficacy in patients (14, 15). The HBT may increase physical activity adherence among people with limited access to gym equipment and facilities. Also, in many countries, HBT has received much attention from the economic point of view as it is a cheaper alternative to GBT (15). Despite this evidence, there are very few studies on the effects of HBT on patients with hypertension, and the answer to the question of whether this type of exercise training can prevent and control metabolic disorders in patients with hypertension requires further investigation.
2. Objectives
The present study aimed at evaluating the effects of combined training in the gym and at home on TXNIP plasma levels, insulin sensitivity, and lipid profile in men with primary hypertension.
3. Methods
3.1. Study Design
This study was a single-blinded randomized controlled trial that used a control group and two experimental groups (combined training groups in the gym and at home). According to the nature of the study, blinding was not possible for intervention practitioners and participants in this study. However, other researchers who were blinded to group assignments performed all laboratory and statistical evaluations. The trial was preregistered in the Iranian Registry of Clinical Trials database (IRCT20160317027092N2). Ethical approval was obtained from the Ethics Committee of Sport Sciences Research Institute (Code: IR.SSRC.REC.1398.108).
All participants completed a research written informed consent form.
3.2. Participants
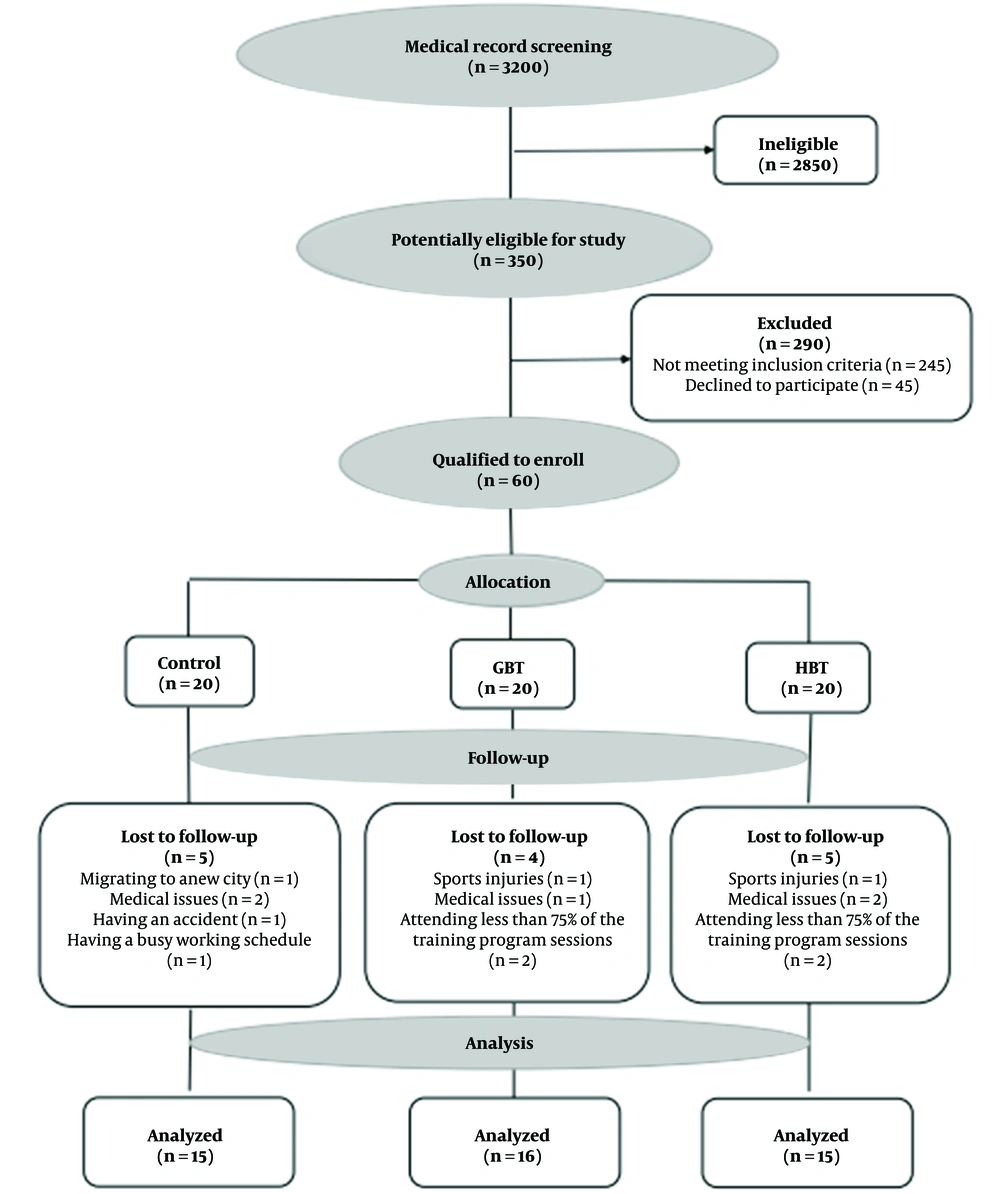
Sixty men with mild systolic and diastolic hypertension who had a medical record in cardiovascular research unit of Dr. Toba Kazemi’s Cardiology Clinic (Birjand, Iran) were selected by purposeful sampling after screening. The inclusion criteria were passing at least one year since the onset of hypertension based on the diagnosis of a specialist physician, participation in regular exercise training, and no need to take calcium channel blocking drugs, such as verapamil (which significantly reduces TXNIP in beta cells) (16), no daily use of more than one type of blood pressure control pills, no history of performing regular exercise and weight loss programs in the six months before the study, no history of cardiac surgery, fatty liver, hyper-or hypothyroidism, diabetes, anemia (Hb < 10 g/dL), kidney disease (creatinine > 1.5 mg/dL), myocardial infarction, heart attack, cardiac arrhythmia, and ischemic disease or unstable chest angina (17), and having SBP and DBP with the ranges of 130 - 159 and 80 - 99 mm Hg, respectively (18). According to these criteria and after age-based homogenization, the individual randomization unit was used. Randomization was done online and through permuted blocks (https://www.randomization.com), with blocks of sizes three and nine used to generate allocation codes. Opaque, sequentially numbered sealed envelopes concealed allocation. Finally, the randomization process was implemented and the participants were randomly sorted into three equal groups (n = 20): control, HBT, and GBT groups. Unfortunately, 14 participants were excluded from the study due to sports injuries, medical issues, changing the dose of the drug or the type of hypertension during the investigation process, refusal to participate in the study, and attending less than 75% of the training program sessions, migrating to a new city, having a busy working schedule, having an accident, and participation in extra exercise training programs. Thus, the final data analyses were done 46 participants (control = 15, GBT = 16, and HBT = 15) (Figure 1).
The required sample size was estimated at 61 participants using G-power software (version 3.1.9.2) (http://www. gpower.hhu.de/en.html) with a power of 0.80 (1-[beta] err prob), alpha error of 0.05, and effect size of exercise training on insulin sensitivity of 0.55 for patients (19).
3.3. Anthropometric, Blood Pressure, Heart Rate, and Aerobic Training Intensity Measurements
Before and after the intervention, weight and height were measured twice in each participant under standardized conditions. Height was measured with the nearest 0.1 cm using BIKI 200 (Jawon Medical Co., Seoul, South Korea), and body weight and BMI were measured following 4-hour fasting using the body composition analyzer (IOI 353; Jawon Medical Co., Seoul, South Korea) by the bio-electrical resistance method. In the next step, a nurse measured the SBP and DBP using a sphygmomanometer (ERKA D-83646 Bad Tölz, Germany) on the right arm after resting in a seated position for 15 minutes.
Participants were instructed to accurately measure resting heart rate or pulse rate (heart rate per minute) a few minutes after waking up while still lying in bed. Also, heart rate during exercise was measured using the manual palpation method (at the wrist). Maximal heart rate (MHR) was measured by the formula: MHR = 220 – age and heart rate reserve (HRR) was calculated by the formula: HRR = MHR – RHR (20). Also, target heart rate (THR) was obtained using the Karvonen Formula: [THR = (HRR × % intensity) + RHR] (20).
To determine the intensity of aerobic exercise training, THR and Borg's rating of perceived exertion (RPE) scale (6 - 20), depending on the choice of subjects, were used. RPE is a reliable indicator for monitoring exercise training intensity. Individuals can subjectively the levels of exertion during exercise using this scale (21).
3.4. Exercise Training Protocols
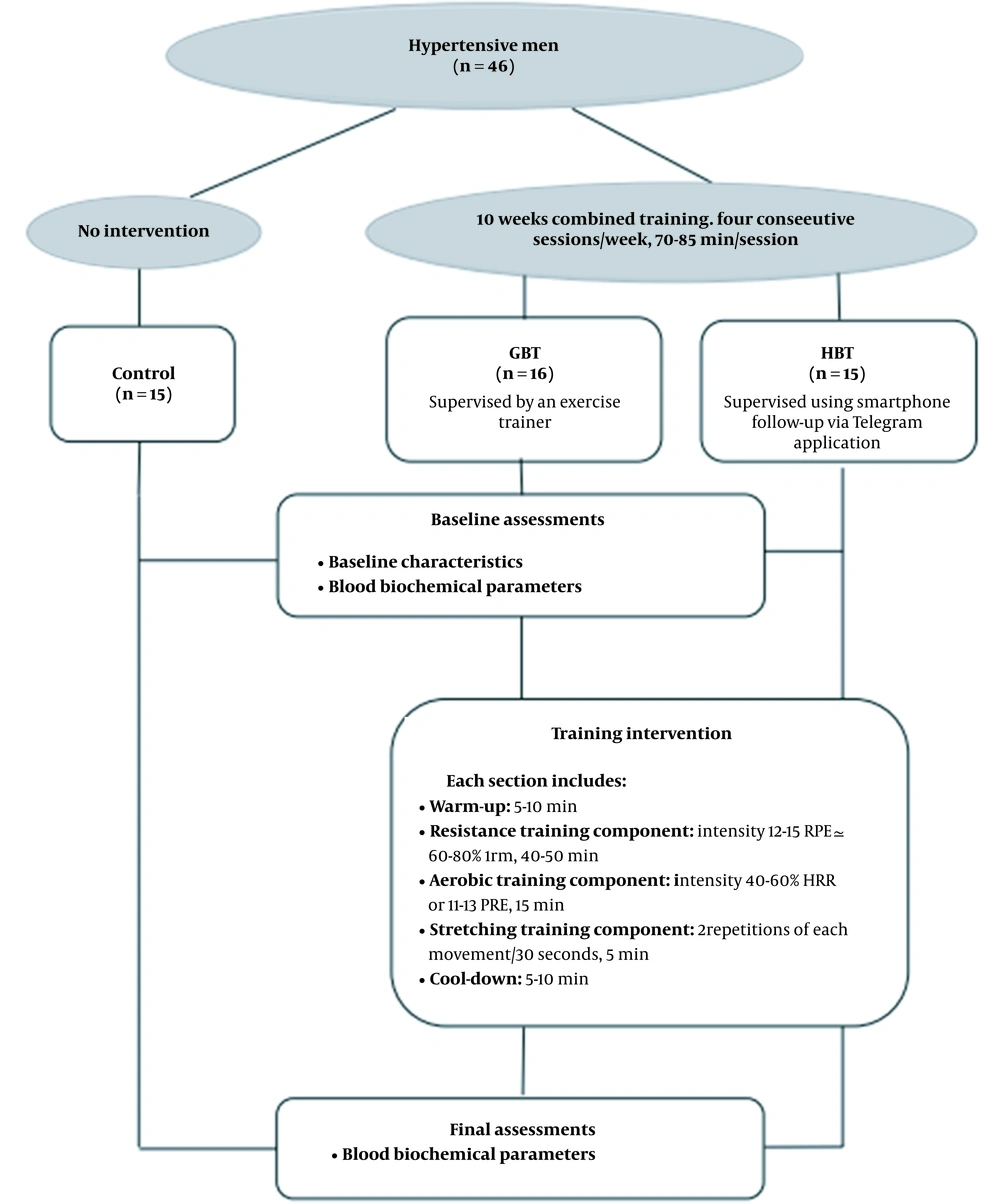
The exercise training intervention was provided in 40 uninterrupted sessions, four sessions per week for ten consecutive weeks. The exercise training protocol was based on the frequency, intensity, time, and type (FITT) principle of the guidelines provided by the American College of Sports Medicine (ACSM) for exercise testing and prescription, 9th Edition (22). The prescribed exercise respectively included resistance (40 - 50 min), aerobic (15 min), and stretching (5 min) exercises in each session. The 5 - 10 min warm-up and cool-down that included stretching and aerobic low-intensity exercise were performed in each session. Participants well tolerated the scheduled exercise training without any adverse events and performed an average of 86.5% of the exercise sessions. Patients in the control group continued their usual lifestyle without any change, such as increasing or decreasing activity, but received usual clinical care and, similar to all of the participants, were under antihypertensive drug treatment (Figure 2).
3.5. The GBT Program
Each exercise session started with resistance training consisting of eight upper-body and lower-body exercises using weightlifting machines (i.e., squat, calf raise, leg press, bicep curl, triceps extension, chest press, lateral pull-down, and scapula retraction). The number of sets, repetitions, and intensity of training was set at 1 - 2, 8 - 12, and 60% of the one-repetition maximum (1-RM; the heaviest load that could be lifted, once only, was calculated using the Brzycki formula (23)), respectively in the initial sessions, which was increased progressively to 2 - 3 sets at 60 ~ 80% of the 1-RM. A one-minute interval was adopted between sets and exercises (15). In addition to resistance training, participants performed aerobic training, including treadmill walking and running or ergometer cycling at an intensity of 40-50% HRR (calculated using the Karvonen formula (24) or 11 - 12 RPE from the first to fifth weeks, and 50 - 60% HRR or 12 - 13 RPE from the sixth to tenth weeks. Finally, stretching training were performed for five major muscle groups, including the chest, shoulder, leg, hamstring, and quadriceps muscles (15). Each muscle group was stretched for 30 seconds in two repetitions.
Trained assistants verbally encouraged the participants and controlled how to perform movements (based on the Health Coaching Australian (HCA) model) (15). Also, to ensure no probable problems with the acute effects of exercise, a nurse was present in the gym in all sessions.
3.6. The HBT Program
The HBT protocol is comparable to GBT, following the American College of Sports Medicine guidelines for chronic health conditions, with resistance, aerobic, and stretching components (Figure 2) (25). It is also similar in the exercise principles (frequency, intensity, time, type, volume, and progression) to the exercise in the gym. Resistance training was performed similarly to the training protocol in the gym (in terms of sets and repetitions) at the intensity of 12 - 15 RPE (60 ≃ 80% 1RM) from 6 - 20 Borg scale, consisting of eight upper-body and lower-body exercises using an elastic resistance band (Thera-Band 038, Guangdong, China) (21). The intensity of the exercise was increased by increasing the stiffness and changing the thera-band elasticity grade (levels of resistance). Participants were instructed to progressively increase resistance after five weeks by advancing to the next level (color) of the elastic band (the pink and blue bands had less and more resistance, respectively). A one-minute interval was adopted between sets and exercises. To perform the aerobic training, the patients were licensed to select a preferred mode of exercise (i.e., walking, ergometer cycling, or treadmill walking and running), followed by 5 minutes of stretching training similar to GBT. The duration and intensity of warm-up and cool-down were similar to the exercise training in the gym.
The HBT program was supervised using smartphone follow-up via Telegram application and telephone calls, based on the Health Coaching Australia (HCA) model (15). The total time spent on supervision for each participant was equal in both treatment groups. The exercise trainers supervising the telephone intervention were the same exercise trainers who provided supervision for the GBT program. Also, the images of the selected movements were provided to the participants in various ways, such as printing images of movements, educational videos, or installation of training programs.
All participants maintained their usual daily activities and took antihypertensive medications according to their physician's advice during the study.
3.7. Blood Sampling and Biochemical Analysis
Blood sampling (5 mL each time) was obtained at 7:00 - 8:00 A.M, after 12 hours of fasting and 24 hours before and 48 hours after the exercise training programs. Venus blood samples were drawn from through the antecubital vein, collected in ethylenediaminetetraacetic acid (EDTA) tubes, and centrifuged at 3000 rpm for 15 minutes. Then plasma was removed and frozen at −80°C for measurements of TXNIP, TG, TC, HDL-C, LDL-C, insulin, and glucose. Plasma concentration of TXNIP was determined using an ELISA kit (made by ZellBio Company, Germany, Sensitivity: 0.5 pg/ml, intra-assay precision: 6.8 %) according to the manufacturer's protocols. The plasma fasting insulin level was assessed using a commercial ELISA kit (Mercodia Company, Uppsala, Sweden, sensitivity: 1 mU/L, intra-assay precision: 5.1 %) according to the manufacturer's protocols. Plasma concentrations of glucose, TG, HDL-C, and TC (enzymatic colorimetric assay) were measured by commercial kits (Pars Azmoon, Tehran, Iran, sensitivity: 1 mg/dL for glucose, TG, and HDL-C and 3 mg/dl for TC). The intra-assay precision, measured as coefficients of variation, was approximately 1.8%, 2.6 %, 5.1%, and 3.1%, respectively. All kits were purchased from Padgin Teb Company (Tehran, Iran). Also, plasma LDL-C was measured using the equation of the standard Friedewald: (LDL-C (SF)= TC-TG / 5-(HDL-C)) (26). The quantitative insulin sensitivity check index (QUICKI) was estimated from the fasting plasma glucose (mg/dL) and insulin (μU/mL) concentrations according to the following formula (27):
3.8. Statistical Analysis
The extracted data were analyzed by SPSS software 26 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean ± standard deviation (SD). The assumptions of normality of the residuals (Shapiro–Wilk test), homogeneity of variance (Levene’s test), and slope of the regression line were examined using the one-way analysis of covariance (ANCOVA). Due to the normal distributions, ANCOVA (TXNIP, insulin sensitivity, HDL-C, LDL-C, and TC) and one-way analysis of variance (ANOVA) (TG and baseline characteristics) followed by the Bonferroni post-hoc test were performed to compare the means between groups. In addition, Pearson’s correlation coefficients were used to determine correlations between variables (TXNIP, insulin sensitivity, insulin, glucose, HDL-C, LDL-C, TC, and TG). The significance level was set at P ≤ 0.05, and the graphs were drawn using GraphPad Prism software version 8.0.1 (GraphPad Software, Inc., La Jolla, CA, USA).
4. Results
Forty-six hypertensive men with an average age of 48 ± 9 years and BMI of 30 ± 4 kg/m2, completed the baseline and ten-week follow-up assessments. Participants’ characteristics are shown in Table 1. There were no significant differences in the characteristics of the research groups at baseline (P > 0.05).
| Variables | Control (n = 15) | GBT (n = 16) | HBT (n = 15) | P-Value |
|---|---|---|---|---|
| Age (years) | 46 ± 9.57 | 50.44 ± 9.33 | 46.00 ± 10.14 | 0.38 |
| Height (cm) | 170.93±7.19 | 169.75±5.79 | 168.80±6.27 | 0.66 |
| Body weight (kg) | 85.12 ± 15.93 | 89.53 ± 18.83 | 86.46 ± 11.73 | 0.73 |
| BMI (kg/m2) | 28.98 ± 4.10 | 30.98 ± 5.82 | 30.17 ± 3.26 | 0.47 |
| SBP (mmHg) | 132.86 ± 12.54 | 144.50 ± 18.63 | 134.20 ± 9.82 | 0.06 |
| DBP (mmHg) | 84.00 ± 8.63 | 86.50 ± 9.22 | 83.40 ± 6.75 | 0.54 |
| RHR (BPM) | 67.40 ±5.85 | 71.06 ± 8.75 | 72.53 ± 5.64 | 0.12 |
Abbreviations: GBT, gym-based training program; HBT, home-based training program; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; RHR, resting heart rate; BPM, beats per minute.
a Data are presented as mean ± SD.
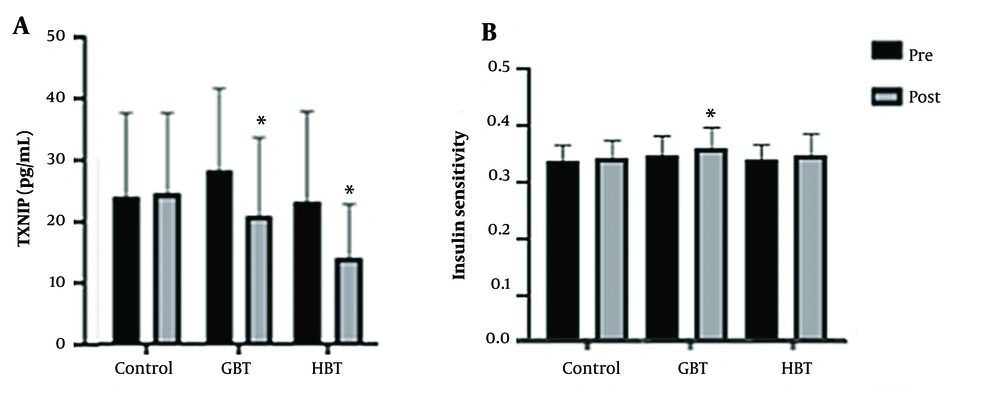
After the intervention period, TXNIP plasma levels significantly decreased in both GBT (P = 0.04) and HBT (P = 0.005) groups compared to the control group. However, no significant differences were found between the GBT and HBT groups (P = 0.99) (Figure 3A).
Insulin sensitivity at baseline and ranges of percent change following intervention are summarized in Figure 3B. The GBT and HBT exerted a positive effect on the QUICKI. However, these increases were statistically significant only following the GBT group compared to the control group (P = 0.01), with no significant difference observed between the GBT and HBT groups (P = 0.14).
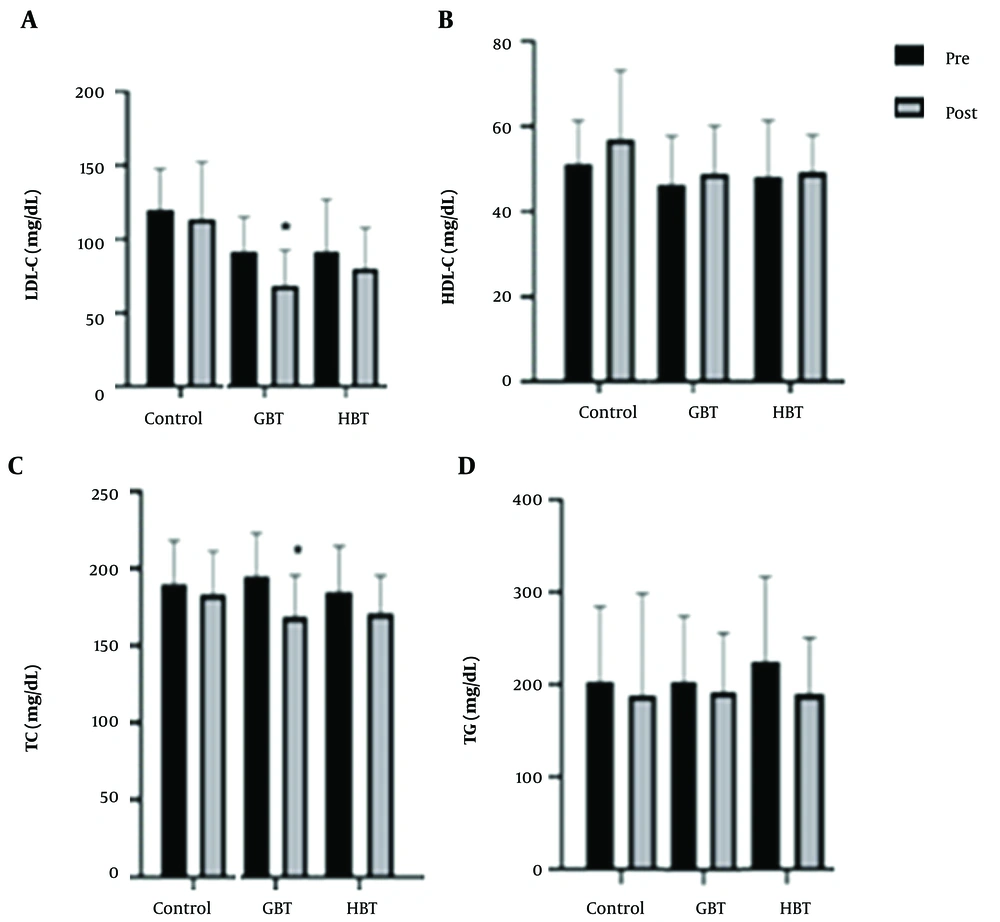
The results of the lipid profile measurements are presented in Figure 4. The GBT and HBT exerted a positive effect on plasma lipid profile. Nevertheless, our study results showed the statistically significant decrease in plasma TC and LDL-C levels only after GBT compared to the control group (P = 0.03 and P = 0.02; respectively), with no significant difference between the GBT and HBT groups (P = 0.47 and P = 0.36, respectively; Figure 4A and C, respectively). We also observed no significant changes in the plasma HDL-C and TG levels after the GBT and HBT compared to the control group (P = 0.20 and P = 0.63, respectively; Figure 4B and D, respectively).
The levels of low-density lipoprotein cholesterol (LDL-C) (A);, high-density lipoprotein cholesterol (HDL-C) (B), total cholesterol (TC) (C), and triglyceride (TG) (D) after ten weeks of the gym-based training (GBT) and home-based training (HBT) programs. Data are presented as mean ± SD. *Significant difference compared to the control group (P ≤ 0.05).
After ten weeks of the GBT and HBT an inverse relationship was identified between the QUICKI with glucose (r = -0.42, P = 0.004), insulin (r = -0.80, P = 0.0001) and TG (r = -0.36, P = 0.01) levels. Also, a positive correlation was found between LDL-C with TC (r = 0.62, P = 0.0001) and glucose (r = 0.38, P = 0.01) and insulin with TG (r = 0.41, P = 0.005) levels (Table 2).
| TXNIP | LDL-C | HDL-C | TC | TG | Glucose | Insulin | QUICKI | |
|---|---|---|---|---|---|---|---|---|
| TXNIP | ||||||||
| Correlation coefficient | -0.10 | 0.16 | -0.10 | -0.12 | 0.07 | 0.05 | -0.04 | |
| P value | 0.52 | 0.27 | 0.49 | 0.40 | 0.65 | 0.72 | 0.79 | |
| LDL-C | ||||||||
| Correlation coefficient | -0.10 | -0.07 | 0.62 a | 0.15 | 0.38 a | 0.09 | -0.15 | |
| P value | 0.52 | 0.60 | 0.0001 | 0.30 | 0.01 | 0.55 | 0.31 | |
| HDL-C | ||||||||
| Correlation coefficient | 0.16 | -0.07 | 0.08 | -0.29 | 0.14 | -0.14 | 0.14 | |
| P value | 0.27 | 0.60 | 0.59 | 0.05 | 0.36 | 0.34 | 0.34 | |
| TC | ||||||||
| Correlation coefficient | -0.10 | 0.62 a | 0.08 | 0.17 | 0.23 | -0.11 | 0.03 | |
| P value | 0.49 | 0.0001 | 0.59 | 0.24 | 0.13 | 0.46 | 0.82 | |
| TG | ||||||||
| Correlation coefficient | -0.12 | 0.15 | -0.29 | 0.17 | 0.05 | 0.41 a | -0.36 a | |
| P value | 0.40 | 0.30 | 0.05 | 0.24 | 0.72 | 0.005 | 0.01 | |
| Glucose | ||||||||
| Correlation coefficient | 0.07 | 0.38 a | 0.14 | 0.23 | 0.05 | 0.20 | -0.42 a | |
| P value | 0.65 | 0.01 | 0.36 | 0.13 | 0.72 | 0.18 | 0.004 | |
| Insulin | ||||||||
| Correlation coefficient | 0.05 | 0.09 | -0.14 | -0.11 | 0.41 a | 0.20 | -0.80 a | |
| P value | 0.72 | 0.55 | 0.34 | 0.46 | 0.005 | 0.18 | 0.0001 | |
| QUICKI | ||||||||
| Correlation coefficient | -0.04 | -0.15 | 0.14 | 0.03 | -0.36 a | -0.42 a | -0.80 a | |
| P value | 0.79 | 0.31 | 0.34 | 0.82 | 0.01 | 0.004 | 0.0001 |
Abbreviation: TXNIP, thioredoxin-interacting protein; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; QUICKI, quantitative insulin sensitivity check index.
a P ≤ 0.05
5. Discussion
In this study, following GBT and HBT, the plasma levels of TXNIP decreased significantly compared to the control group. TXNIP reduction rate was somewhat higher in the HBT group, but a statistical analysis indicated no significant difference between the two experimental groups. It has been shown that aerobic exercise training increases TRX1 and decreases TXNIP in mononuclear cells and skeletal muscle of adult male C57B6L mice (28). Increasing TRX expression independently generates an imbalance in the TRX/ TXNIP ratio, decreasing their relative interaction and leading to an enhanced antioxidant capacity and ROS scavenging (10).
Since oxidative stress plays an important role in increasing TXNIP, exercise training in the gym and at home possibly decreases TXNIP by modulating oxidative pressure. However, since this study did not evaluate oxidative stress indices, these results cannot be relied upon with certainty. In this regard, Mota et al. showed that combined training (aerobic and strength training) reduced oxidative stress and damage caused by oxidative stress, and improved functional capacity in women over 40 years (29). The results of Niebauer et al. supported the beneficial effect of HBT on reduced oxidants and increased antioxidant power in patients with heart failure (30). Also, four weeks of stretching training significantly decreased malondialdehyde-modified low-density lipoprotein cholesterol (MDA-LDL), ROS, and oxidative stress in chronic heart failure patients. They reported that skeletal muscles under mechanical stretch increased the expression of antioxidants in skeletal muscle cells. On the other hand, stretching training, through increased the expression of antioxidants, such as NO in vascular endothelium, improved vascular endothelial dysfunction (31). Because the presence of NO significantly reduces the expression of TXNIP (32) it seems that combining these three types of training (aerobic, resistance, and stretching) is more useful than either aerobic, resistance, or stretching training alone. On the other hand, blood glucose and lipid profile include factors that increase oxidative stress. Elevated glucose levels by increasing carbohydrate response element-binding protein (ChREBP) and enhanced TXNIP activity lead to beta-cell apoptosis (2, 33). Therefore, the exercise by lowering blood sugar and oxidative stress may reduce TXNIP levels. In addition, Szpigel et al. suggested that plasma TXNIP levels are directly associated with plasma TG. Therefore, exercise-induced reduction of blood lipids is another mechanism of TXNIP reduction (33).
The results of the present study showed that the levels of plasma TC and LDL-C decreased and insulin sensitivity increased following GBT. Consistent with these results, Arazi et al. showed that eight weeks of combination exercise training (resistance and aerobic), positively improved TG, TC, LDL-C, HDL-C, and blood glucose levels in middle-aged men with cardiovascular risk symptoms. In this study, it was suggested that the decrease in TG may be due to increased activity of the enzyme lipoprotein lipase. Combination exercise training (resistance and aerobic), unlike resistance training, seems to increase the activity of this enzyme (8). Also, exercise training may reduce cortisol levels and arousal thresholds by modulating sympathetic nerve activity, leading to stabilizing the hypothalamic-pituitary-adrenal axis and increasing the balance of the autonomic nervous system, which accelerates the cellular process of glucose and fat metabolism (34). The regular exercise increases the expression of lipolysis, beta-oxidation, Krebs cycle, and electron transfer chain enzymes and mitochondrial density which cause weight loss and BMI reduction (35). Also, Attarzadeh Hosseini et al. also indicated that eight weeks of combination exercise training (resistance and aerobic) significantly improved insulin, glucose levels, and body fat percentage in middle-aged men. In this study, among the mechanisms that increased post-workout insulin action was increased insulin receptor signaling, glucose transporter proteins (GLUT-4) expression, skeletal muscle blood flow, and glycogen synthase activity and hexokinase enzymes. Since skeletal muscle is the main site of glucose uptake in the normal state and isometric contractions lead to insulin-like effects on glucose uptake in isolated skeletal muscle, it can be assumed that increased skeletal muscle mass resulting from exercise training can be an effective intervention to improve insulin sensitivity (36).
We demonstrated that the HBT had a positive effect on plasma lipid concentrations and insulin sensitivity, but the result was not statistically significant, which is in line with earlier studies, where no significant effect was found for the levels of LDL-C, HDL-C, TC, and TG following 12 weeks of HBT (aerobic training at 60% target heart rate) in people with epilepsy (37). In contrast, Chen et al. showed that a three-month HBT (stepping and cardio-dance) improved the levels of HDL-C in men and women with metabolic syndrome (38). Also, Tiainen et al. investigated the effect of a two-year HBT (five endurance-based sessions and one strength-based session per week at a 50–80% target heart rate intensity) with different loads (high, medium, and low) in coronary artery disease patients and showed that only higher-volume training resulted in the decreased concentration of oxidized LDL (ox-LDL), with no difference between the two groups performing lower-volume training (39). The differences between the results of studies may be due to various factors, including the duration of the intervention, individual differences, intensity and load of training, and the variety of exercise training protocols (39).
As reported earlier in the current study, insulin sensitivity increased in hypertensive patients after GBT but not HBT; however, we did not observe any between-group differences. This finding is similar to the results of the previous studies indicating HBT with no important impact on the insulin sensitivity index in women with gestational diabetes in men and women with metabolic syndrome (40). Also, Dunstan et al. assessed the effect of a 12-month high-intensity progressive resistance exercise training (six months of GBT followed by six months of HBT) in men and women with type 2 diabetes. They indicated improvements in glycemic control after the first six months of interventions of supervised GBT. However, this effect was not maintained in additional six months of HBT. It seems that supervised GBT is more effective than HBT therapy (41). In our study, the HBT group possibly had poorer adherence to the determined HBT program compared to the GBT. Therefore, HBT may need exercise supervision to improve biochemical variables.
However, low-cost telemonitoring exercise interventions that are easily integrated into clinical practice and patient lifestyles have great potential for patients (14).
On the other hand, the association between TXNIP and insulin sensitivity is also important. Parikh et al. showed that TXNIP reduces insulin sensitivity and increases blood glucose levels. TXNIP inhibits glucose uptake by increasing the oxidative metabolism of pyruvate and inhibiting glycolysis. Deletion of TXNIP causes metabolic reprogramming towards glycolysis and increases glucose uptake (42). Immobilization and disuse (i.e., reduction of muscle contractile activity) quickly enhance TXNIP, simultaneously with a decline in insulin-independent and insulin-dependent glucose uptake (9). Therefore, it seems that participating in a regular and codified exercise program can play a major role in reducing TXNIP and improving insulin sensitivity (42). TXNIP also induces miR-204 by interdicting the activity of signal transducer and activator of transcription 3 (STAT3), a transcription factor that is involved in the regulation of miR-204. The Maf family of transcription factors (MafA) is a target that is down-regulated by miR-204 and plays a very important role in insulin secretion. MafA is an insulin-like gene transcription factor and regulator of other glucose-sensitive genes in pancreatic beta cells (43). Hasanpour et al. showed that 12 weeks of aerobic (running on the treadmill) and resistance (running on the treadmill with a weight attached to the animals’ tails) exercise training in the male Wistar rats with type 2 diabetes significantly increased MafA expression and reduced fasting glucose levels (44). These results demonstrate that TXNIP–miR-204– MafA –insulin pathway may contribute to diabetes advancement and provides new insight into the function of TXNIP and microRNA biology in health and disease (43, 44). Gorgens et al. indicated that 12 weeks of combined strength and endurance exercise training under hypoxia in individuals with type 2 diabetes, reduced TXNIP (as a negative regulator of insulin action) expression by increasing the protein levels of hypoxia-inducible factor 1α (HIF1α). HIF1α is a key factor for insulin action and glucose metabolism in skeletal muscle that down-regulates the TXNIP expression (45). Since exercise training decreases TXNIP expression in muscle, and muscle TXNIP mRNA expression negatively correlates with the glucose secretion rate; therefore, it improves insulin signaling and glucose metabolism in human subjects (45).
In conclusion, this study provides further insights into the key role of TXNIP in the molecular regulation of fat metabolism and insulin sensitivity in skeletal muscle after exercise training (with emphasis on comparing the GBT and HBT protocols). However, the present study did not measure the enzyme activity and concentration of antioxidants, lipolysis enzymes, or glycolysis. Whether combined training in the gym and at home elevate intracellular levels of antioxidants should be clarified in future studies. Moreover, further investigation is necessary to assess whether combined training in the gym and at home cause up-regulation of hexokinase enzyme and lipoprotein lipase activity in skeletal muscles. In addition, the apparent ineffectiveness of the HBT in improved plasma lipid profile and insulin sensitivity was most likely due to a reduction in adherence and direct monitoring during the home-based training. Because maintenance of optimal glycemic control is one of the basic factors in preventing the spread of type 2 diabetes following hypertension, more studies involving other types of approaches to increase commitment and continuous monitoring of exercise training protocols at home are warranted. Also, because we know that this study was completed using a small sample size and there was no other available sample to replace missing individuals, it may limit the detection of statistical significance for some outcomes. Therefore, additional studies with larger sample sizes are needed to obtain more accurate findings and verify the clinical usability of these exercise training programs.
5.1. Limitations
There were several limitations in this study. An important limitation of the study was the sample size due to the lack of more available subjects. Also, the conditions and training environment for the training group at home were different and uncontrollable. It was also not possible to use male and female participants because of the intervening effect of estrogen changes (e.g., contraceptive methods, menopausal status, and stages of the menstrual cycle) on TXNIP levels (46)
5.2. Conclusions
The effectiveness of GBT is slightly more compared to HBT in the regulation of blood glucose and improving the lipid profile. Also, HBT improves some important biochemical parameters; therefore, it can be a suite of complementary strategies instead of GBT. Our data manifest a positive effect of combined exercise training on reducing the protein expression of molecular targets that negatively impact glucose and lipid metabolism in hypertensive men. These findings give a new insight into the mechanisms of the GBT and HBT for reducing diabetes risk in hypertensive men.