1. Background
Chronic kidney diseases (CKDs) manifest as progressive and irreversible renal dysfunction. From 2003 to 2016, the incidence of end-stage renal disease (ESRD) was relatively constant in many developed countries, but it increased significantly in East and Southeast Asia. The five-year survival rate of ESRD patients on hemodialysis has been estimated as 41% in the United States, 48% in Europe, and 60% in Japan (1).
In Iran, large population-based studies are scarce, and existing surveys have shown marked heterogeneity in CKD prevalence in the general population (2). In a study by Najafi et al., the prevalence of CKD stage III+ was reported to be 8.9% (3). In another study, this rate was reported as 23.8% in individuals with ages higher than 40 years in Golestan province, north of Iran (4). Whether this increase is due to a true rise in CKD prevalence or other factors, including the type of study, contributing to this finding needs to be clarified.
Patients with ESRD may also have some complications, the most important of which are cardiovascular events (5). The risk of mortality due to cardiovascular complications in hemodialysis patients is 10- to 30-fold higher compared to the general population (5). Other complications include hypertension, electrolyte disturbance, and bone disorders secondary to hyperparathyroidism and vitamin D deficiency, as well as hyperuricemia, metabolic acidosis, hyperphosphatemia, hypoalbuminemia, and anemia (6). Vascular access for dialysis is accompanied by some complications, including bleeding, localized or diffuse intravascular infections, and fistula obstruction. Electrolyte imbalance after dialysis, dementia, and dialysis imbalance syndrome are among other noteworthy complications observed in dialysis patients (7).
Various risk factors play a role in the pathogenesis of cardiovascular diseases (CVDs) in hemodialysis patients, including peri-dialysis fluid overload, contributing to the development of left ventricular hypertrophy (LVH) and heart failure. Fluctuations in the plasma levels of sodium, potassium, calcium, and magnesium during hemodialysis can lead to dreadful cardiac arrhythmias. In addition, hypotension during dialysis can predispose to ischemic events in various organs and tissues, such as the myocardium, intestine, and central nervous system, which often remain clinically undetectable. The role of agents such as cardiotonic steroids (CTS) and Na+/K+ pump inhibitors in the management of these events is also under investigation. The serum levels of ouabain, marinobufagenin, telocinobufagin, and bufalin are significantly elevated in patients with ESRD (8).
Pulmonary hypertension (PH) is one of the most common complications among various disorders, including ESRD, and is characterized by a gradual increase in pulmonary artery pressure (PAP). Pulmonary hypertension is the main cause of right heart failure among patients with CVDs. In addition, PH is a serious threat to the life of ESRD patients (9).
The incidence of PH among patients with stage 5 CKD has been reported between 9% and 39%, and this rate varies from 8.8% to 68.8% among ESRD patients who undergo hemodialysis. The incidence of PH among patients treated with peritoneal dialysis has been noted to be up to 42% (10). A study conducted in Iran reported PH in 62.3% of CKD patients who were on maintenance hemodialysis (11).
The hormonal and metabolic disorders associated with ESRD increase PAP and, in combination with vascular resistance, result in PH. The small bubbles created during dialysis may be trapped in the pulmonary artery, predisposing to PH. In addition, left-heart diseases (including left ventricular hypertrophy, left ventricular diastolic and systolic dysfunction, and mitral valve regurgitation), elevation in cardiac output caused by arteriovenous fistula (AVF), alveolar-capillary membrane destruction, chronic hypoxic and obstructive pulmonary diseases (such as sleep apnea), endothelial dysfunction, anemia, fluid overload, and other factors may trigger the development of renal failure-associated PH (12).
Pulmonary hypertension is divided into two categories: Primary (or idiopathic) and secondary (13). Several factors may play a role in the occurrence of PH, including age, sex, increased arteriovenous fistula flow, decreased ejection fraction (EF), parathormone hormone, fluid overload, smoking, malnutrition, mineral bone disease, and using non-biocompatible dialysis membranes (14). Malnutrition is one of the common adverse effects of hemodialysis in ESRD patients, and its prevalence has been reported to be 40 - 70% (15).
Suboptimal nutrition is common in patients with CKD and ESRD and is the main cause of protein malnutrition. Poor nutritional status has been associated with multiple adverse metabolic and physiological changes, including metabolic acidosis, altered intestinal flora, and hormonal disturbances, all of which can accelerate the progression of kidney disease and increase morbidity and mortality (16).
Patients suffering from CKD and ESRD often have superimposed diseases and should adhere to nutritional and medical restrictions. If the energy supply is suboptimal in these patients, followed by a rapid loss of lean body mass due to protein degradation, leading to increased morbidity and mortality (17).
In these patients, nutritional status and malnutrition are assessed by laboratory criteria such as serum levels of albumin, triglyceride, cholesterol, transferrin, pre-albumin, amino acids, creatinine, urea, C-reactive protein (CRP), and protein equivalent of nitrogen appearance (PNA), as well as blood sugar and anthropometric measures, including body mass index (BMI) (18). Of course, no specific method has yet been universally validated to evaluate malnutrition (19). Some studies have noted a relationship between the nutritional status and a reduction in serum albumin, triglycerides, cholesterol, and BMI, as measures of malnutrition in hemodialysis patients, predicting an increase in the prevalence of PH (20).
Regarding the high prevalence of malnutrition and PH in hemodialysis patients, investigating the prevalence of this condition and its associated factors can be helpful in controlling some complications in these patients (17). The early detection and management of PH and identifying the factors associated with this condition can help prevent or at least mitigate pathophysiological changes, comorbidities, and the course of the disease in hemodialysis patients (21). Also, early interventions can prevent the aggravation of heart failure and reduce mortality due to PH (22). Considering that PH is an important cause of mortality and morbidity in hemodialysis patients, investigating the prevalence of PH in these patients and divulging its relationship with parathyroid hormone can play a significant role in improving the quality of life of patients, decreasing the disease severity, reducing its financial burden, extending patient survival, and better managing comorbidities.
2. Objectives
This study aimed to determine the prevalence of PH in hemodialysis patients and its association with age, gender, BMI, duration of dialysis, anemia, serum albumin level, and lipid profile.
3. Methods
3.1. Study Design
In this cross-sectional study, all hemodialysis patients referred to the Kowsar Medical Center from 1 March 2016 to 31 August 2016 were enrolled by the complete enumeration sampling method. Inclusion criteria were age older than 18 years and undergoing at least three months of hemodialysis through a fistula or a graft. Patients with chronic heart failure (CHF) (ejection fraction, EF < 35%), mitral and aortic valve diseases, lung disease (including diseases related to the chest wall, lung parenchymal disease, history of pulmonary embolism and chronic bronchitis), and previous history of sleep apnea were excluded from the study. Eligible patients underwent echocardiography the day after dialysis, and coronary artery parameters were recorded. Pulmonary hypertension was defined as PAP higher than 35 mmHg (18).
Patients with PH were evaluated on the day after dialysis by a pulmonologist to check underlying lung disease through chest X-ray, PFT, and CT scan of the lungs, and all patients, even those with PH secondary to lung and cardiovascular diseases, were enrolled in the study and evaluated for nutritional status. In order to check laboratory parameters, blood samples were collected during dialysis and sent to the laboratory.
Demographic data, including age, gender, duration of hemodialysis, and values of laboratory tests (hemoglobin, albumin, total protein, triglycerides, cholesterol), as well as the adequacy of dialysis (through BUN measurement before and after dialysis), type of vascular access, and anthropometric data (i.e., weight, height, arm circumference, and body mass index related to the post-dialysis dry weight of patients) were entered in a datasheet. Patients were classified into two groups based on their nutritional status: Sufficient nutritional status (albumin > 3.8 g/L, cholesterol < 100 mg/dL, triglyceride < 150 mg/dL, body mass index > 20) and insufficient nutritional status (albumin < 3.8g/L, cholesterol > 100 mg/dL, triglyceride > 150 mg/dL, body mass index < 20) (23). Demographic data and laboratory test results were compared between patients with and without PH.
3.2. Data Analysis
The data were analyzed by SPSS software version 21. Numerical independent variables were reported as mean, and standard deviation, and regression models were used to calculate the odds ratio (OR). Quantitative variables were compared between the groups by the independent t-test, and the chi-square test was used for qualitative variables. Logistic regression was used to ascertain the likelihood of PH in patients with low albumin and hemoglobin. The significance level was considered less than 0.05.
4. Results
In this study, 80 hemodialysis patients referred to the Kowsar Medical Center, Semnan, Iran, were evaluated for PH, of whom 32 (40%) patients had PH based on echocardiography findings. The relationship between PH and other variables was evaluated.
Among patients with PH, 62.5% were male, and 37.5% were female. There was no significant difference in the incidence of PH between the two genders (P = 0.500). The mean of age ( ± SD) in patients with and without PH was 65.84 ± 14.00 and 61.02 ± 14.63 years, respectively, showing no significant difference between the two groups according to the independent t-test (P = 0.132, Table 1).
| Variables | Pulmonary Artery Pressure | P-Value | |
|---|---|---|---|
| High, No. (%) | Normal, No. (%) | ||
| Gender | 0.500 | ||
| Male | 20 (62.5) | 32 (55.2) | |
| Female | 12 (37.5) | 26 (44.8) | |
| Albumin | 0.077 | ||
| Normal (3.5 - 5.5 g/dL) | 9 (28.1) | 23 (47.9) | |
| Low ( < 3.5 g/dL) | 23 (71.9) | 25 (52.1) | |
| Hemoglobin | 0.008 | ||
| Normal (> 11 g/dL) | 12 (37.5) | 35 (60.3) | |
| Low (< 11 g/dL) | 20 (62.5) | 23 (39.7) | |
| Age, mean ± SD (y) | 65.84 ± 14.00 | 61.02 ± 14.63 | 0.132 |
| Transferrin, mean ± SD (mg/dL) | 362.63 ± 85.68 | 359.14 ± 85.59 | 0.854 |
| Duration of dialysis, mean ± SD (y) | 4.13 ± 3.33 | 3.09 ± 2.73 | 0.115 |
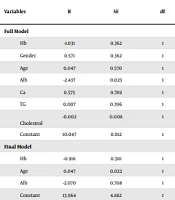
Among patients with PH, 23 (71.9%) had low serum albumin levels (below 3.8 g/L), and nine (28.1%) patients had normal albumin levels (3.8 g/L and above). In patients with normal PAP, 25 (52.1%) individuals had hypoalbuminemia, and 23 (47.9%) had normal serum albumin levels. According to a binary logistic model, the likelihood of PH in those with low albumin increased by almost 2.3-fold compared to those with normal albumin levels (P = 0.003, Table 2).
| Variables | B | SE | df | P-Value | Odds Ratio | 95% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Full model | |||||||
| Hb | -1.031 | 0.362 | 1 | 0.004 | 0.357 | 0.175 | 0.726 |
| Gender | 0.571 | 0.362 | 1 | 0.316 | 1.770 | 0.579 | 5.405 |
| Age | 0.047 | 0.570 | 1 | 0.043 | 1.048 | 1.001 | 1.096 |
| Alb | -2.437 | 0.023 | 1 | 0.002 | 0.087 | 0.019 | 0.411 |
| Ca | 0.575 | 0.789 | 1 | 0.147 | 1.777 | 0.818 | 3.861 |
| TG | 0.007 | 0.396 | 1 | 0.374 | 1.007 | 0.992 | 1.023 |
| Cholestrol | -0.002 | 0.008 | 1 | 0.901 | 0.998 | 0.975 | 1.023 |
| Constant | 10.047 | 0.012 | 1 | 0.090 | 23085.999 | ||
| Final model | |||||||
| Hb | -0.916 | 0.310 | 1 | 0.003 | 0.400 | 0.218 | 0.735 |
| Age | 0.047 | 0.022 | 1 | 0.033 | 1.048 | 1.004 | 1.094 |
| Alb | -2.070 | 0.708 | 1 | 0.003 | 0.126 | 0.031 | 0.506 |
| Constant | 13.964 | 4.882 | 1 | 0.004 | 1159594.489 | ||
Twenty (62.5%) of patients with PH had low hemoglobin levels (below 11 g/dL), and 12 patients (37.5%) had normal hemoglobin (11 g/dL and above). In patients with normal PAP, 35 (60.3%) had low hemoglobin, and 23 (39.7%) had normal hemoglobin. The two groups were significantly different in terms of the frequency of low hemoglobin levels (chi-square, P = 0.003, Table 1). In the binary logistic model, the likelihood of PH in those with normal hemoglobin decreased by almost 0.4-fold compared to those with low hemoglobin (Table 2).
The mean ± SD of serum transferrin in patients with PH was 362.63 ± 85.68 mg/dL, which showed no significant difference compared to patients with normal PAP (359.14 ± 85.59 mg/dL, P = 0.854). The duration of dialysis also showed no significant difference between patients with PH (4.13 ± 3.33 years) and without PH (3.09 ± 2.73 years, P = 0.115) (Table 1).
The means ± SDs of BMI and arm circumference were not significantly different between patients with or without PH (P = 0.219 and P = 0.713, respectively). The means ± SDs of serum cholesterol (P = 0.252) and triglyceride (P = 0.773) levels were comparable between the two groups of patients with or without PH. Finally, serum BUN levels before and after dialysis showed significant differences between patients with or without PH (P < 0.001).
5. Discussion
Out of 80 hemodialysis patients studied, 32 were diagnosed with PH, indicating a prevalence of 40%. There was no significant association between PH and age, gender, dialysis duration, BUN before dialysis, EF, TG, cholesterol, transferrin, albumin, and anthropometric measures.
Malnutrition is one of the common complications of ESRD patients undergoing hemodialysis, with a prevalence of 40 - 70% (19). Suboptimal nutritional intake is common in CKD and ESRD patients and poses a direct risk for protein malnutrition. Poor nutritional status has been associated with several adverse metabolic and physiologic changes, including metabolic acidosis, altered intestinal flora, and hormonal disturbances, all of which can expedite the progression of CKD, increasing related morbidities and mortality (24).
As’habi et al., who evaluated the nutritional status of hemodialysis patients using the subjective global assessment (SGA) method, noted that in hemodialysis patients suffering from type I malnutrition, there was no increase in unexpected risk factors for CVDs, while in type II malnutrition, two inflammatory markers associated with cardiovascular events, serum sICAM-1 and CRP levels, were reported to be elevated (25). Rashidi et al. reported that an increase in the frequency of dialysis had an effect on the nutritional status of hemodialysis patients so that with an increase in the number of dialysis sessions, the nutritional status would improve in hemodialysis patients (26).
Chertow reported that the prevalence of malnutrition (defined based on albumin < 3.8 g/dL, pre-albumin < 3 mg/dL, and cholesterol higher than 100 IU, as well as BMI, anthropometric measures, and serum protein) in patients with ESRD was between 40-70% and suggested periodic assessment of nutritional status in these patients (19). In our study, the prevalence of PH was significantly associated with hemoglobin levels. In the binary logistic model, it was estimated that the risk of developing PH in those with normal hemoglobin (11 g/dL and higher) decreased by almost 0.298-fold compared to individuals with low hemoglobin levels.
Findings regarding the effects of erythropoietin-stimulating agents on the risk of PH in hemodialysis patients are inconsistent (27). In a study recruiting a small sample size, intravenous erythropoietin administration increased peripheral vascular resistance in subjects with PH, as well as in healthy individuals (28). Experimental models have shown that erythropoietin can prevent PH (29); on the other hand, some studies have indicated a positive association between erythropoietin and PH (28). Mimura et al. reported that prescribing high-dose erythropoietin to achieve the target hemoglobin increased the risk of CVD while maintaining hemoglobin above 12 g/dL without exogenous erythropoietin stimulating agents did not pose a risk for developing CKD. Therefore, evidence opposed high-dose erythropoietin to achieve the target hemoglobin level (30).
Mahdavi-Mazdeh et al. reported a prevalence of 51.6% for PH (PAP > 35 mmHg) in hemodialysis patients. Hemoglobin and serum albumin levels were significantly lower in hemodialysis patients with PH than in those without PH, and PH was inversely associated with EF and directly with dialysis duration (22). Domenici et al. evaluated PAP in dialysis patients and concluded that the duration of dialysis, level of hemoglobin, and serum albumin were higher in patients with PH compared to patients with normal PAP (31). Bolignano et al. reported an association between anemia and increased PAP, exaggerated by the exacerbation of hypoxia (32). Tarrass et al. evaluated PAP by echocardiography in dialysis patients and did not report any relationship between PH and either age, gender, duration of dialysis, vascular access location, biological parameters, or PTH level (33). Regarding the association of PH with malnutrition and body structure in hemodialysis patients, Genctoy et al. pointed out that there was a relationship between serum triglyceride, cholesterol, and albumin levels, as well as BMI and PH in hemodialysis patients (17).
Mukhtar et al. reported in their study that PH was more common in females and in patients with longer duration of dialysis, as well as in those with vascular access such as arteriovenous fistulas; however, there was no significant relationship between age and PH (34). Dagli et al., by considering a 30 mmHg cut-off for PAP, noticed a significant relationship between the incidence of PH in hemodialysis patients and increased arterio-venous fistula flow, lower left ventricular EF, and smoking (35). In the study of Sedighi et al., who also considered a 35 mmHg cut-off point for PH diagnosis, there was a correlation between neither hemoglobin, calcium, phosphorus, albumin, alkaline phosphatase, triglyceride, cholesterol, nor parathyroid hormone and pulmonary hypertension; however, there was a significant correlation between the duration of dialysis through arteriovenous fistulas and the incidence of PH (14).
AlAhmad et al. reported that the incidence of PH was high among ESRD and stage 5 CKD patients, being reported as 21.8%. In the recent study, the development of PH was not associated with gender, duration of dialysis, hemoglobin, creatinine, albumin, phosphorus, calcium, parathyroid hormone, type of vascular access, and shunt site (36).
In our study, the prevalence of PH in hemodialysis patients was obtained as 40%. In general, the prevalence of PH in hemodialysis patients is high and varies between 20% and 70%. In Nagaraju et al.’s study, the prevalence of PH was 54%, and the majority of the cases were mild (9). The reason for this discrepancy in the prevalence of PH can be attributed to ethnic diversity, the presence of comorbidities, and diagnostic criteria used in different studies. Despite the fact that these studies used different diagnostic criteria, most of them reported a high prevalence of PH among hemodialysis patients (10).
The pathogenesis of PH in CKD is multifactorial. Although definite risk factors have not been recognized for this condition, some risk factors noted include age, cardiac disorders, dialysis condition, arteriovenous fistulas, dialyzer membranes, fluid overload, chronic anemia, mineral bone disease, uremic toxins, and untreated uremic vasculopathy (37). However, several studies have not reported any correlation between PH and age or gender (9, 33). Some studies have pointed out a lack of association between PH and diabetes, hypertension, smoking, cerebral vascular accident (CVA), and ischemic heart diseases (IHD) (10).
Some studies have indicated an association between AVF-assisted hemodialysis and the development of PH (38-40). Nevertheless, different opinions exist on the effects of AVF on the risk of PH. Yigla et al. (40) demonstrated that increased cardiac output after AVF grafting could boost PAP. Afzal et al. (38) showed that AVF was one of the causes of unexplained increased PAP and might contribute to the development of PH. On the other hand, other studies reported no association between AVF and PH development. Overall, most evidence suggests that AVF is not a key determinant in the risk of PH (41).
Mehta et al. (42) reported a higher prevalence of PH in patients with a longer duration of dialysis, and Smukowska-Gorynia et al. (43) reported a decrease in PH immediately after dialysis, which was attributed to ultrafiltration. However, in our study, the duration of dialysis had no effect on the occurrence of PH. Consistent with previous studies, we observed that laboratory parameters other than hemoglobin (i.e., albumin, transferrin, cholesterol, triglyceride, and BUN) were not associated with PH in hemodialysis patients.
Some studies have investigated the effect of inflammatory factors and oxidative stress on PAP. Nagaraju et al. found no significant relationship between PH and inflammatory factors (e.g., CRP), oxidative stress (MDA), and thiol levels in hemodialysis patients (9). On the other hand, Sonkar et al. (44) declared elevated levels of an inflammatory marker (alpha-1-acid glycoprotein) in hemodialysis patients with PH. A study by Smukowska-Gorynia et al. (43) showed elevated levels of MDA in patients with PH; however, MDA levels were comparable between non-CKD individuals and CKD patients who were not under hemodialysis. This difference can be explained by ethnic and genetic variabilities among patients, as well as various diagnostic markers employed in different studies.
Nagaraju et al. spotted a significant difference in some echocardiographic parameters between patients with or without PH. Besides, left and right ventricular dysfunction was significantly higher among patients with PH (9). This was similar to the findings of Agarwal (45) and Ramasubbu et al. (46), indicating a multifactorial mechanism for PH, encompassing increased mean arterial pressure, hypoxemic stress (due to anemia), cardiac myocyte dysregulation (caused by uremia), chronic volume overload, and impaired heart function (due to micro- and macro-vascular dysfunction). All these elements can lead to the subclinical impairment of left and right ventricular function, boosting pulmonary capillary wedge pressure and ultimately causing PH.
Among the potential factors involved in the occurrence of PH are mitral insufficiency and left ventricular (LV) systolic dysfunction. Secondary mitral regurgitation to various degrees results from structural and functional LV changes. The long-term elevation of LV end-diastolic pressure (LVEDP) is recognized as a key factor in the development of PH. Therefore, secondary mitral regurgitation and PH seem to have common causes in patients with CKD (40).
As discussed earlier, PH in CKD can occur through several mechanisms. In our study, hemoglobin was an effective factor in the occurrence of PH. The risk of having elevated blood pressure is almost 2.5 times higher in patients with hemoglobin levels higher than 11 gr/dL compared to people with hemoglobin levels less than 11 g/dL.
Oygar and Zekican reported significantly low levels of hemoglobin in patients undergoing hemodialysis and suffering from PH (P = 0.01). Anemia can be a factor in the development of left ventricular hypertrophy (LVH) and increased cardiac output, so it may contribute to the pathogenesis of PH (47). In our study, the results were in favor of this hypothesis, where the mean hemoglobin level in hemodialysis patients with PH was significantly lower than their counterparts without PH. However, some studies have reported inconsistent results, showing no correlation between hemoglobin level and PAP (33).
In our study, the likelihood of PH was more than twice in patients with lower levels of serum albumin than in patients with normal levels of albumin. Nickel et al. mentioned a relatively higher prevalence of low-grade albuminuria in patients with PH. However, no association was found between albuminuria and right heart hemodynamics, which could be related to systemic inflammation and insulin resistance (48). Cerik et al. referred to the applicability of the CRP/albumin ratio as a simple marker in determining the prognosis of PH (49).
Pulmonary arterial hypertension (PAH) is a debilitating disease with a complex underlying mechanism that leads to increased pulmonary vascular resistance, right ventricle (RV) overload, and, ultimately, the risk of RV failure and death (50). Particularly, PAH is characterized by non-specific signs and symptoms, which causes delays in the early diagnosis and timely treatment of this condition. Current diagnostic tools, such as right heart catheterization and echocardiography, are very invasive (51), and serum albumin measurement can help diagnose PAH and evaluate the inflammatory state by predicting the degree of pulmonary macrophage infiltration. Accordingly, this parameter has been proposed as a new non-invasive diagnostic and monitoring tool in PAH (52).
In a study by Mahdavi Mazdeh et al., hemodialysis patients suffering from PAH had lower levels of hemoglobin and serum albumin compared to their peers without PAH (22). Sedighi et al. reported a prevalence of 44% for PAH in patients under chronic hemodialysis and pointed out that no significant relationship was found between PAH, anemia, hyperparathyroidism, hypoalbuminemia, and dyslipidemia (14). These contradictory results can be attributed to the effects of nutritional factors on the changes in hemoglobin and serum albumin, affecting the prevalence of anemia and hypoalbuminemia in these patients.
Snipelisky et al. evaluated the predictive value of serum albumin in patients with PAH and showed that lower albumin levels were associated with more adverse outcomes in the presence of other systemic diseases and reflected faster disease progression (53). This finding was consistent with studies conducted in other populations (54-57).
Albumin is the most abundant protein in the blood and is exclusively synthesized in the liver and is subsequently secreted into plasma, entering both intra‐ and extravascular spaces. Serum albumin levels are influenced by several factors, including nutritional and inflammatory status. Albumin breakdown occurs in intravascular compartments. In patients with heart failure, the transcapillary escape rate of albumin may increase, which is associated with an increase in right atrial pressure (58). In animal studies, it has been stated that hypoalbuminemia increases the permeability of pulmonary vessels, resulting in pulmonary edema (59). In patients with PAH, endothelial dysfunction and capillary escape are common phenomena and have been hypothesized to contribute to the loss of plasma proteins (56, 60, 61).
Serum albumin participates in several pathophysiological processes that can be involved in PAH progression, and hypoalbuminemia may be a non-specific risk marker for advancing PAH. Liver dysfunction, malnutrition, and systemic inflammation may ensue progressive right ventricular failure caused by PAH, which in the presence of hypoalbuminemia, reduces the synthesis of albumin by the liver, worsens kidney and liver function, and lowers hemoglobin levels (62-65). Although kidney and liver dysfunction, as well as inflammation and malnutrition, can contribute to decreasing levels of albumin, its independent association with multi-organ dysfunction provides more evidence of the systemic effects and burdens of PAH.
In animal studies, increased albumin concentrations in bronchoalveolar lavage samples in rats with PAH indicated the presence of vascular albumin losses as a potential cause of hypoalbuminemia (66). Therefore, low serum albumin levels may be due to increased capillary permeability and subsequent loss of albumin into the intravascular space rather than solely decreased hepatic production of this protein. Capillary dysfunction is an integral component of the underlying pathophysiology of PAH, so it is possible that the degree of capillary dysfunction correlates with the degree of hypoalbuminemia. In addition, albumin degradation can occur in the intravascular space, possibly due to endothelial dysfunction and capillary leakage (64).
Other studies have investigated the relationship between serum albumin levels and the clinical outcome of PAH, as a secondary endpoint, in smaller populations. For example, Kawut et al. showed that higher albumin levels, as well as warfarin use, cardiac index, and acute vascular reactivity, were independently associated with improved survival in patients with PAH (67). Haddad et al. examined outcome predictors in patients with acute decompensated PAH and revealed an association between hypoalbuminemia and increased in-hospital mortality, a higher respiratory rate, renal dysfunction, hyponatremia, and more severe tricuspid regurgitation, as other important markers of the systemic effects of PAH. Haddad et al. also confirmed the importance of hypoalbuminemia as a predictor of outcomes in patients with PAH (68).
5.1. Conclusions
Pulmonary hypertension in CKD can occur through several mechanisms. In our study, anemia seemed to be an independent risk factor in the occurrence of PH. The risk of PH was observed to increase in hemodialysis patients with low serum albumin and hemoglobin levels compared to counterparts with normal values for these parameters. So, it is very important to prevent anemia and maintain hemoglobin levels at the normal range for these patients during treatment.
5.2. Study Limitations
The patients enrolled in our study were mainly patients with a poor prognosis and had a low level of cooperation, especially for echocardiography. In some cases, we needed to repeat echocardiography. The sample size of our study was small; it is recommended to conduct multicenter studies with a larger number of patients. Overall, based on our and other studies’ results, it is recommended to conduct more studies in this field.