1. Context
Food additives are substances that are usually used to prepare and process all sorts of food to give favorable features. Accordingly, it is a substance that is added to the food to enhance its flavor, appearance, or other favorable qualities (1). the food protection committee of the US national research council described food additives as “a substance or a mixture of substances other than a basic foodstuff that is present in a food as a result of an aspect of production, processing, storage, or packaging” (2). Following the US FDA (Food and Drug Administration), a food additive is “any substance, the intended use of which results or may reasonably be expected to result–directly or indirectly–in it becoming a component or otherwise affecting the characteristics of any food” (3) Additives are mostly included as synthetic chemicals. Present-day consumers are turning to natural substances and bio-based additives due to the adverse effects caused by some chemicals (1). food additives based on the official UK food standards agency consist of colors, preservatives, antioxidants and acidity regulators, thickeners, stabilizers and emulsifiers, anticaking agents, flavor enhancers, antibiotics, glazing agents and sweeteners, and additional chemicals (4).
Under the US FDA, “A color additive is any dye, pigment, or substance, which when added or applied to a food, drug or cosmetic, or the human body, is capable (alone or through reactions with other substances) of imparting color” (3). Food color is primarily used as a food additive to enhance sensory effects, such as visual satisfaction. There are two sorts of food colors, certified colors, and colors excluded from certification. The certified colors are synthetic compounds. They are usually more effective than natural mixtures and they do not introduce off-flavors to the foods. Colors determined from natural sources are excluded from certification. These compounds are more costly than synthetic compounds. However, the colors excluded from certification may grant off-flavors to the food (3).
In recent years, a wide variety of food additives have been used to improve the quality, stability, flavor and price of food (5). Artificial food additives include azo, xanthene, triphenylmethane, quinoline, and indigotin dyes (6). Azo dyes, the largest group of synthetic dyes, are identified by one or more azo groups (N=N) in their structure and aromatic rings linked to them. Some of these aromatic rings are cleaved into aromatic amines, which are toxic and carcinogenic (7, 8).
Azo dyes are used for coloring food products such as candies, jam, citrus marmalade, custard powders, orange sodas, energy drinks, ice cream, packet soups, and chips (6, 9). Despite the low cost, stability, availability, and uniformity of azo food dyes, excessive consumption can result in various side effects in children, especially due to low body weight (10). The adverse effect of azo dyes has been investigated for decades. It has been reported that azo dye agents, especially food color additives, can cause hypersensitivity, hyperactivity, and learning disabilities in children (11). Elbanna et al. reported that artificial food colorants might cause significant adverse effects on the liver, spleen, kidney, small intestine, and, stomach, suggesting that oxidative stress can be induced by the toxic metabolism of azo dyes (12). Several azo dyes, such as tartrazine, Sunset yellow, Sodium benzoate, Sudan III, ponceau 4R, Metanil yellow, erythrosine B, Chocolate brown HT, and Allura red, negatively induce neurobehavioral defects and brain function disorders (13-17). This review aimed to evaluate the effect of the azo dyes used for food coloring on brain tissues.
2. Food Additive Azo Dyes
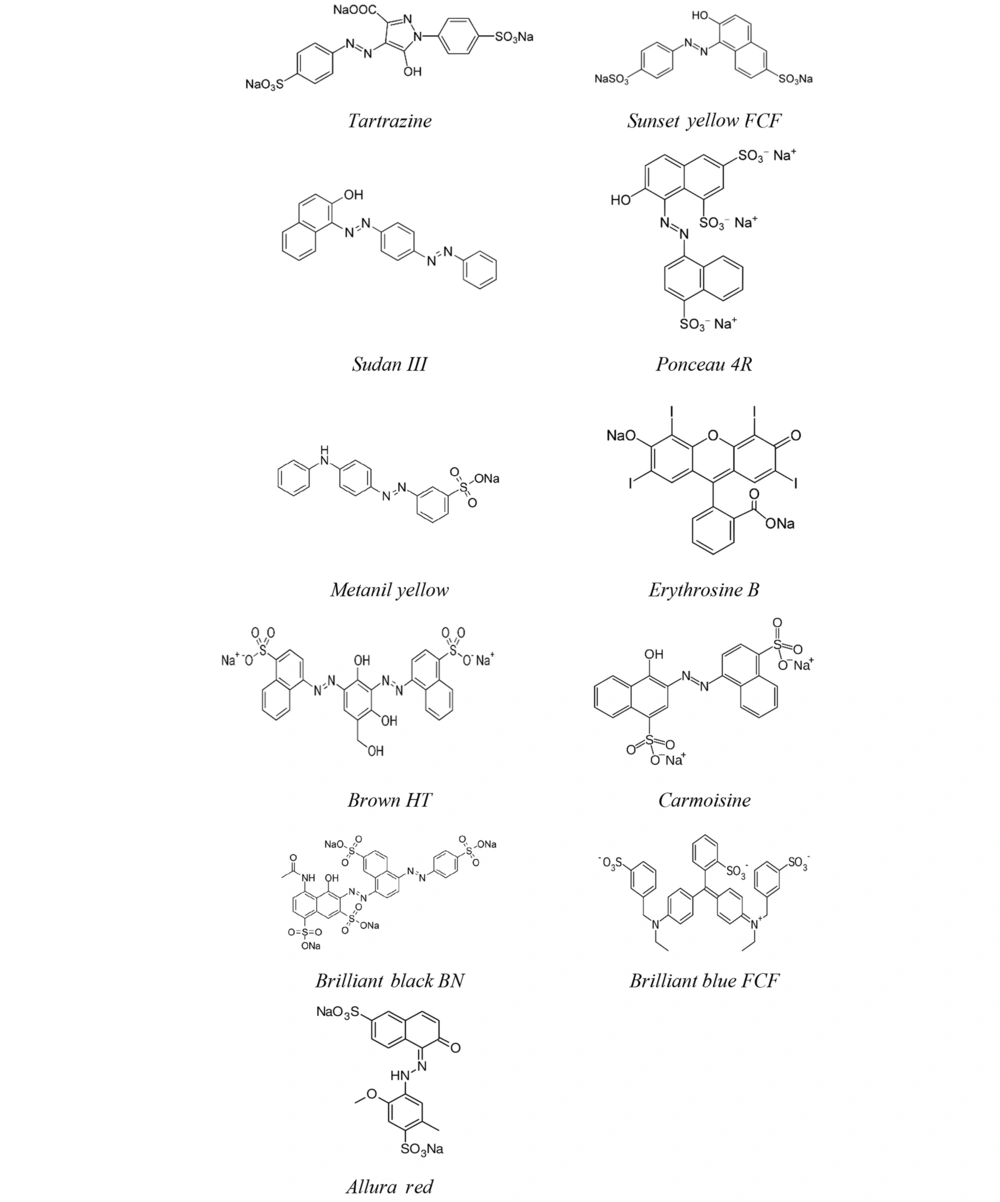
Food additive azo dyes have been used in the food and pharmaceutical industries for many years because they increase the quality of the food and preserve its appearance and nourishing food sources. In particular, high amounts of azo dye can cause various side effects such as attention deficit hyperactivity disorder (ADHD), learning disability, and memory disorder. This paper reviews the knowledge and research advances related to the toxicity of several azo dyes on brain sub-regions. A summary of a list of Azo dyes with their properties that are applied in the food industry can be seen in Figure 1 and Table 1. Figure 1 and Table 1 represents a list of popular Azo dyes used in the food industry with their properties, codes (courtesy of chemblink website (18)), chemical formula, structure (courtesy of ChemSrc website (19)) , color, solubility, molecular weight (g/mol), and acceptable daily intake (ADI)(mg/kg BW/day) courtesy of European food safety authority (EFSA) (20).
| Names | Codes, and Chemical Formulas | Color | Solubility | MW (g/mol) | ADI |
|---|---|---|---|---|---|
| Tartrazine | (E102, C16H9N4Na3O9S2, C.I. 19,140, FD&C Yellow 5, Acid Yellow 23) is a component, lemon yellow, water solvent color dye, which is obtained from coal tar (12). | Lemon yellow | Water | 534.36 | 0 - 7.5 |
| Sunset Yellow FCF | (E110, C.I. 15,985, Orange Yellow S, FD&C Yellow 6, C16H10N2Na2O7S2) is an orange-yellow azo dye and is used in different types of food (12). | Yellow | Water and ethanol | 452.37 | 0 - 4 |
| Sudan III | (Solvent red 23, C.I. 26100, C22H16N4O) a member of the Sudan dyes family which has confirmation for use in cosmetic products (12). | Reddish brown | Water, benzene, chloroform | 352.39 | Not noted |
| Ponceau 4R | (E124, C.I. 16,255, Cochineal Red A, C.I. AcidnRed 18, C20H11N2Na3O10S3) a water-solvent powder and strawberry red azo color which is usually utilized in drinks, syrups, sweets, jelly beans, sugar candy, ice cream, and other foods (12). | Red | Water and ethanol | 604.47 | 0 - 4 |
| Metanil yellow | (Acid Yellow 36, Sodium 3Acid Metanil Yellow, C18H15N3NaO3S) is used widely as a food supplement which is synthesized from diphenylamine and diazotized metanilic acid. | Red to yellow (depending on PH) | Water | 375.38 | 0 - 0.3 |
| Erythrosine B | (E 127, Red No. 3, C20H6I4Na2O5) is a unique agent with a polyiodinated xanthene structure which is approved by the US Food and Drug Administration (FDA). | Pink | Water, spirit, methanol, methyl cellosolve, and ethanol | 879.86 | 0 - 0.1 |
| Brown HT | (E155, Chocolate Brown HT, Food Brown, C27H18N4Na2O9S2) is used in ice cream, soft drinks, piddles, flour confectionery, sauces, sugar candy, and additives (Food Additives and Contaminants Committee, 1979). | Brown | Water | 652.56 | 0 - 1.5 |
| Carmoisine | (E122, Azorubine, Food Red 3, C20H12N2Na2O7S2) has an aromatic structure and is usually found in the form of disodium salt (FDA). | Red to maroon | Water | 502.43 | 0 - 4 |
| Brilliant Black BN | (E151, C.I. Food Brown 1, C28H17N5Na4O14S4) | Black | Water | 867.68 | 0 - 1 |
| Brilliant blue FCF | (E 133, BBG, C.I. 42090, C37H34N2Na2O9S3) is a water-soluble, functional, and structural analog of FD&C blue dye No. 1 and is widely utilized as a coloring agent and food additive. | Blue | Water | 792.85 | 0 - 6 |
| Allura Red | (E129, Food Red 17, C.I. 16,035, C18H14N2Na2O8S2) The foremost common red colorant determined from Azo is Allura ruddy (Amin K, 2010), and is widely used in candies, ice cream, drinks, and bakery products. | Red | Water | 496.42 | 0 - 7 |
Tartrazine (E 102, FD, and C Yellow). The formula of tartrazine dye (Yellow 5) is C16H9N4Na3O9S2, which is usually a solid orange-colored powder at room temperature (21). Tartrazine is a component, lemon yellow, water solvent color dye, which is obtained from coal tar (22). After tartrazine dye consumption, the body loses zinc, an element that is vital for the proper function of cognition (23). It has been shown that Yellow 5 might be involved in the deficits of cognition, memory, and learning in mice and rats (21-23) (Figures 2 and 3). Also, it has been suggested that tartrazine exposure at the fetal level significantly affects cognitive flexibility and the organism's memory (21). Exposure to Yellow 5 leads to an increase in the level of malonaldehyde, which is an oxidative stress marker and decreases the antioxidant enzymes in the rat brain (22, 24). The administration of tartrazine dye was associated with a decrease in the level of 5-hydroxytryptamine in the cerebellum. This phenomenon might be correlated with the synthesis of free radicals after the metabolization of azo food color by gastrointestinal bacteria. The radical oxygen inhibits the generation of adenosine triphosphate (ATP), which leads to the decreased synthesis or reabsorption of the transmitter in the presynaptic neuron (25). Previous studies suggest that the probable mechanism for tartrazine-induced oxidative stress is due to the aromatic ring liked by an azo bond to the second naphthalene or benzene ring that turns into aromatic amines (i.e., Sulphanilic acid) after oral ingestion from intestinal bacteria and the liver's cytosolic and microsomal enzymes (26-28), Sulphanilic acid then enters the brain cells through the blood-brain barrier and causes oxidative stress by producing hydroxyl radical, hydrogen peroxide and superoxide anions through its metabolism (29, 30) Previous experimental studies have shown that exposure to tartrazine has effects on brain performance and accompanies by constructive changes in the prefrontal cortex and cerebellum (22, 31). Based on past literatures, in animals treated with tartrazine compared with the controls, no crucial change in the brain weight was observed whereas body weight gain, and tissue protein levels in all brain sub-regions were decreased (31). It has been noted that among all the brain sub-regions alterations with tartrazine, the cerebellum and the frontal cortex were the most prone to the oxidative stress caused by the metabolic production of Sulphanilic acid as a mechanism of its toxicity, also tartrazine caused a significant increase in levels of Malondialdehyde (MDA) in all brain sub-regions which shows the damage due to reactive oxygen species (31). Another study found that perinatal exposure to tartrazine within the acceptable daily intake (ADI) was associated with neurobehavioral changes in animal models (32). Moreover, neuronal degeneration of the cerebrum, chromatolysis, and pyknosis 21 and 35 days after exposure of mice offspring to different doses of tartrazine was seen (32) (Figure 4). Also, apoptotic and anti-apoptotic properties of tartrazine at low and high concentrations were confirmed in the liver of mice, respectively (33). Moreover, tartrazine elevates the level of gamma-aminobutyric acid (GABA) through the inhibition of the calcium channels. According to the previous description, it is suggested that antioxidants should be used for the side effects of tartrazine. Vitamin E is suggested to be used as a therapeutic and neuroprotective agent in neurodegenerative diseases (23).
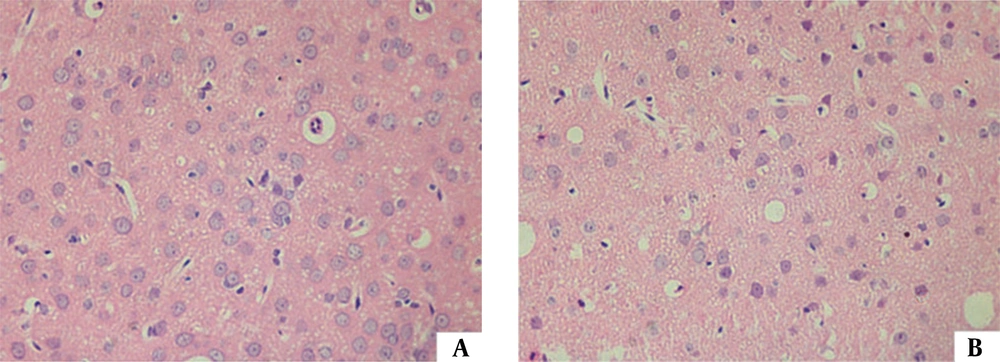
Histopathological changes in rat brain with H&E stain. A, Control rats; B, Rats with tartrazine consumption. These changes include swelling, vacuolar degeneration, karyopyknosis and apoptosis (23)
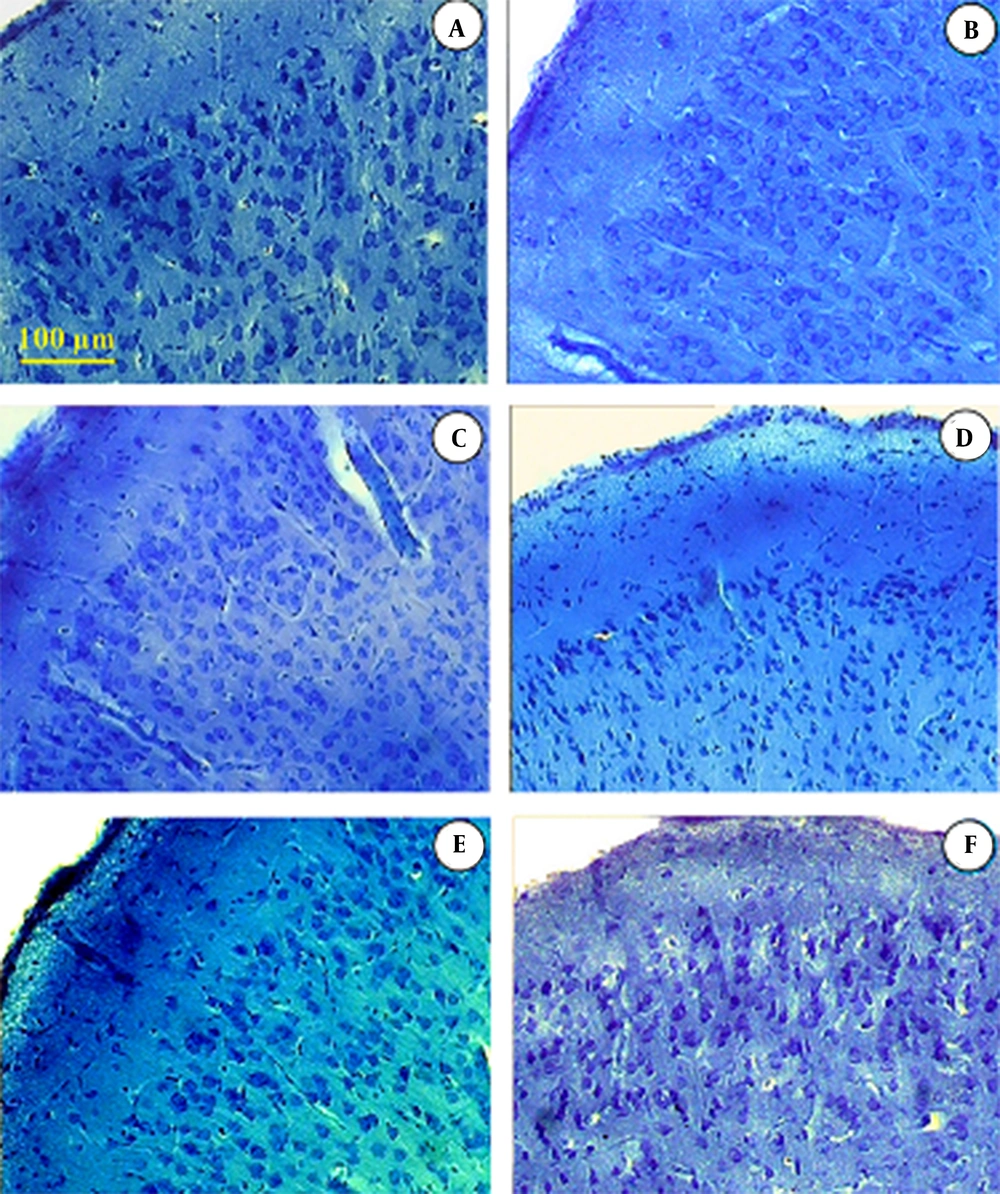
Giemsa staining of the cortex of cerebrum of mice (23).
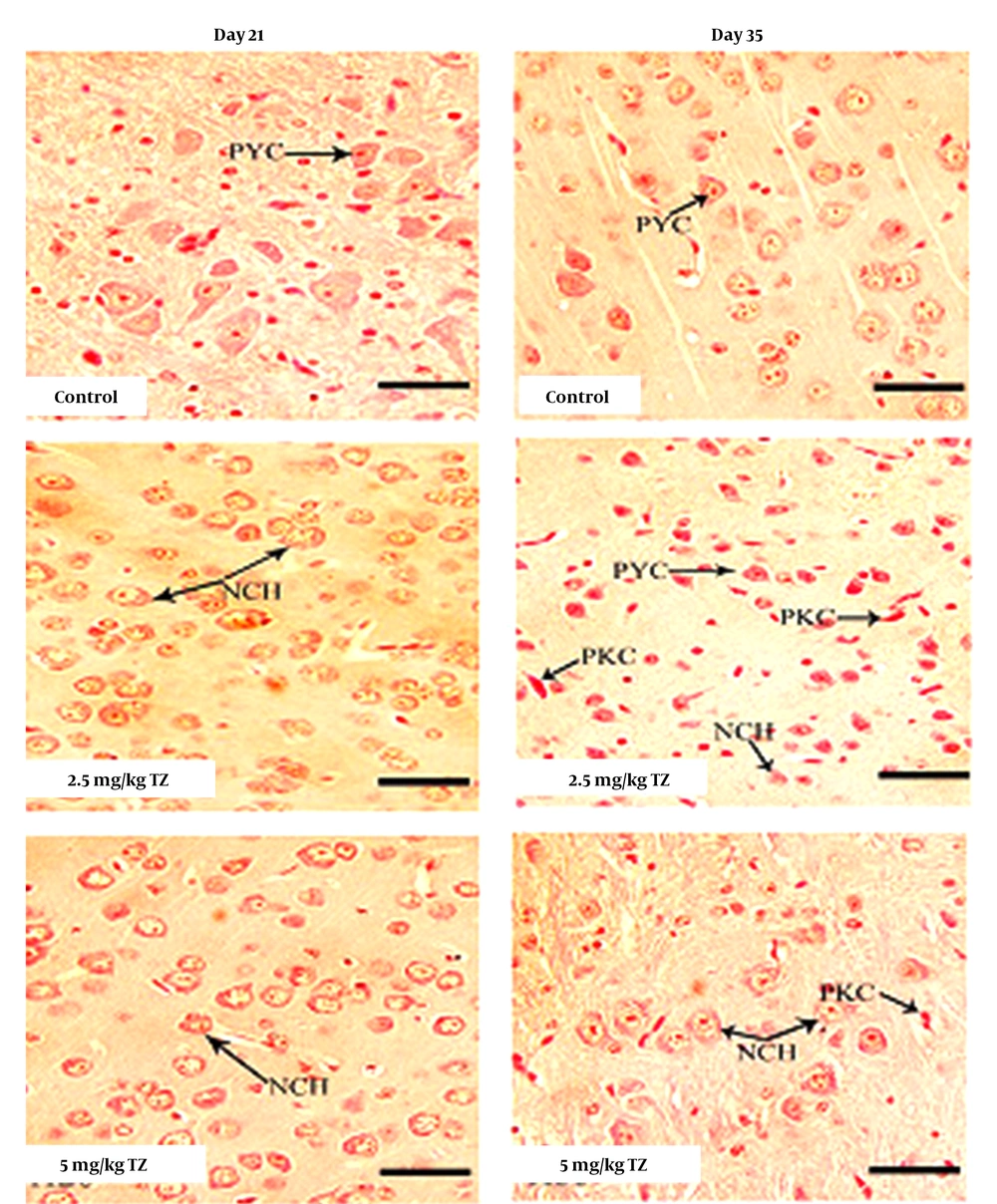
H&E stain sections of the cerebral cortex of newborn mice at days 21 and 35. Control mice showing normal histological structure and pyramidal cells. However perinatal exposure to tartrazine induced neuronal chromatolysis and pyknosis (32).
Sunset yellow (E 110, molecular weight 452.36). Sunset yellow, which is an orange-yellow azo dye, is approved as a food colorant in India (34). This food colorant is used in different types of foodstuffs such as jams and jellies, candies, sweets, canned juice, pickles, sauces, ice cream, and many other food products (35, 36). Studies have shown that sunset yellow significantly increases chromosomal aberration (Gap, breaks, ring, and delayed) (37). The primary lesion in the brain due to the sunset yellow is characterized by the aggregation of mononuclear cells along with congestion and dilation of the meningeal blood vessels (Figure 5) (34). A previous study revealed that consumption of sunset yellow induced the generation of free radicals, which subsequently increased the concentration of malondialdehyde in the kidney, brain, and liver tissues (34). This study showed a significant reduction in the SOD activity in rats treated with sunset yellow (38). It has been reported that post-weaning exposure to sunset yellow induces structural changes in the adult rats medial prefrontal cortex and behavioral impairment (39). Sunset yellow administration decreases the glutathione (GSH) concentration; this finding was in the same line with the results reported by previous studies (40, 41). Sunset yellow can also induce meningioma due to the lack of this organic diet in biodegradation (34).
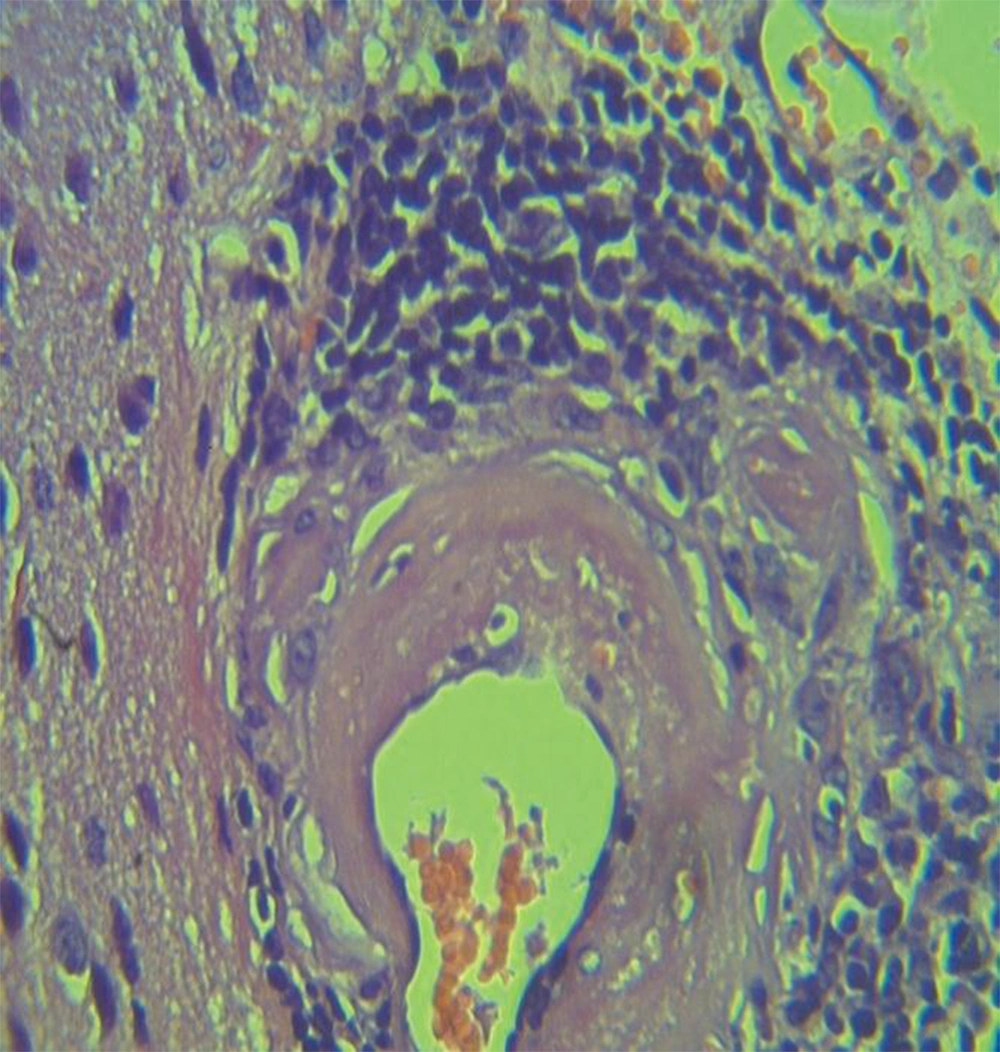
Histopathological section in the brain of animal treated with Sunset yellow FCF 8weeks post treatment with H&E stain shows mononuclear cells aggregation severe dilation and congestion meningeal blood vessels (34).
Sodium benzoate (E 211, molecular weight 144.10). Sodium benzoate (SB) is a popular water-soluble food additive that has fungi static and bacteriostatic properties. This agent is broadly utilized in different food preparations, including jellies, pickles, jams, carbonated drinks, etc. FDA has allowed the use of SB in food products at a limit of 0.1% (1000 ppm) (42). It has been suggested that SB might be correlated with childhood hyperactivity (43), angioedema (44), asthma (45), urticaria (46), and other behavioral disorders (47, 48). Non-toxic amounts of SB can inhibit cell defense responses (49). It has been shown that SB can damage memory and initiate oxidative status in mice with increased malondialdehyde and decreased glutathione concentrations in the brain, which was statistically significant (50). SB can also induce nephrotoxicity and neurotoxicity in zebrafish larvae; however, there is not enough information in the field on oxidative stress and behavior due to SB exposure (51, 52). Some previous investigations have reported that SB might be neuroprotective due to its effect on overexpression of protein deglycase DJ-1 and neurotrophic factors (53-55). It has been hypothesized that SB may modify neurological disease symptoms through other mechanisms (56). It is important to note that higher sodium benzoate intake may be correlated with attention deficit hyperactivity disorder. (ADHD)-related symptoms in pediatrics (48, 57). Conversely, in another study, SB could protect the ability of learning and memory due to reducing oxidative stress in the hippocampus by increasing the GSH level and decreasing the homocysteine level (58).
Sudan III (Solvent red 23, CI 26100). Sudan dyes, an azo and diazo colors, are chemical materials widely utilized as colorants in foods, solvents, textiles, waxes, cosmetics, etc. The Sudan III is a member of the Sudan dye family, which has confirmation for use in cosmetic products that do not contact the mucus membranes due to its lipophilicity (EC, 2005; MERCOSUR, 2008, 2011). Sudan III can induce enzymes that metabolize drugs to suppress the toxic effects evoked by 7,12-dimethyl-Benz (a) anthracene (DMBA) in vivo. Hatakeyama et al. demonstrated a statistically significant assuagement of DMBA-induced reticulocytes in mice administered Sudan III (59). Hatakeyama et al. also revealed that Sudan III initiated activities of 7-ethoxycoumarin O-demethylase, CYP1A1, glutathione S-transferase, and uridine 5´-diphosphoglucuronic (UDP)-glucuronyl transferase (59). Moreover, it could increase DMBA mutagenicity when hepatic microsomes from rats treated with Sudan III were used in the Ames. It has been shown that Sudan III can stimulate a ligand-activated transcription factor, which is named the aryl hydrocarbon receptor (AhR). This receptor can mediate the biological and toxic effects of different compounds (60). Moreover, the decrease in this component can lead to the renal carcinogenicity of estrogens (61).
Ponceau 4R (E 124, molecular weight 604.46). Ponceau 4R (1-(4-sulpho-1-napthylazo)-2-naphthol-6,8-disulphonic acid, trisodium salt), which is a water-solvent powder and the strawberry red azo color, is usually utilized in drinks, syrups, sweets, jelly beans, sugar candy, ice cream, and other foods (62-65). It is recommended that humans consume P4R and SY up to a maximum of around 2.5 and 4.0 mg/kg body weight, respectively (66). Momma et al. revealed that the presence of Ponceau 4R in the meals during pregnancy in mice did not have any teratogenic or postnatal development effect (67). In previous studies on the effect of ponceau 4r with and without Vitamin E treatment it’s been stated that body weight gain reduces with P4R due to reduced food utilization (68) or vitamin C deficiency (69) whereas vitamin E treated groups had no significant change in weight gain (70, 71). According to previous studies, there was no significant difference in the percentage of brain weight/body weight in P4R and P4R with Vitamin E or natural antioxidant groups (70, 72). It has been noted that P4R may cause Acetylcholinesterase inhibition causing cholinergic poisoning due to metabolic alterations of brain cholinergic synapses (70, 73, 74).
Metanil yellow (Acid Yellow 36, molecular weight 375.38). Metanil yellow, as a yellow azo dye, is used widely as a food supplement. This chemical component is synthesized from diphenylamine and diazotized metanilic acid. It has been confirmed to be used in coloring wool, silk, nylon, paper, aluminum, cleanser, ink, and so on; however, due to its toxicity, metanil yellow is not allowed to be used in nourishment materials. It has been shown that metanil yellow induces ROS in different vital organs, including the liver, kidneys, and heart (75, 76). This component can lead to deficits briefly in the kidneys, liver, heart, intestines, gastric tissue, nervous tissue, and all organ system of humans (75-79). It has been demonstrated that oral administration of metanil yellow can extraordinarily influence the amine levels in definite zones of the brain, including the brain stem, hypothalamus, and striatum. It is necessary to mention this point that the withdrawal of metanil yellow administration was not accompanied by a reversal of the adverse changes in neurotransmitter concentrations (80). The administration of metanil yellow also adversely influenced learning ability (80). Previous investigations have demonstrated that exposure to metanil yellow is accompanied by harm in both Purkinje cells and the granular layer of the brain (80, 81).
Erythrosine B (E 127, molecular weight 879.86). Erythrosine B (ErB, 20,40,50,70- tetraiodofluorescein), which has a polyiodinated xanthene structure, is a cherry-pink food colorant. Erythrosine B is a unique agent with a polyiodinated xanthene structure, which is approved by the US Food and Drug Administration (FDA). Thus, this food colorant is widely utilized in drugs, cosmetics, and foods (82-84). In the European Union, erythrosine B is utilized in a wide range of foods such as biscuits, sweets (e.g., Turkish delight), sausages, and glace and tinned cherries (85). It is suggested that erythrosine B is involved in the learning disabilities and hyperkinesis of children. Lafferman and Silbergeld (86) have shown that this agent inhibits dopamine transfer into animal caudate presynaptic components. Also, Logan and Swanson showed that a combination of seven food colorants inhibited the aggregation of eight neurotransmitter precursors or neurotransmitters by rat brain homogenate (87). Erythrosine B was the only food colorant that prevented the aggregation of Dopamine neurotransmitters. It is important to note that the effective amount of erythrosine B was as low as 1 µg/mL. Mailman and Lewis revealed that this agent inhibited the transport of dopamine into rat striatum presynaptic components (88). However, dopamine transport was associated with the number of existing synaptosomes. All of these were due to the interaction of erythrosine B with neuromuscular membranes (89) and neuronal membranes (90). Mekkawy et al. conducted an investigation in which male rats were treated with erythrosine B (with supplemented diets of 0.08 and 0.4 g/kg) for 30 days. The measurement of the chromosomal variations of the bone marrow and the concentrations of nucleic acids and total protein in the brain and liver demonstrated changes in mutagenic exercises (91).
Chocolate brown HT (E 155, CI 20285). Chocolate brown HT benefits are in ice cream, soft drinks, piddles, flour confectionery, sauces, sugar candy, and additives (Food Additives and Contaminants Committee, 1979). It was shown that chocolate brown HT led to a statistically significant reduction in noradrenaline quantity in all of the experimental areas after 2, 3, and 4 weeks. The highest reduction in noradrenaline quantity was discovered in the brain stem, striatum, hippocampus, and cerebellum. A minimal decrease in dopamine levels was seen in the brainstem, hypothalamus, and striatum after 4 weeks. Chocolate brown HT led to a statistically significant reduction in GABA contents which were initiated in the hippocampus, cerebral cortex, hypothalamus, brain stem, and striatum from the second to fourth week. T The greatest reduction in GABA content was seen in the hypothalamus and striatum. Chocolate brown HT led to a statistically significant reduction in the serotonin quantity which was initiated from the third and four weeks in all brain areas. The highest reduction in the contents of serotonin was found in the hypothalamus and striatum after four weeks. Oral intake of chocolate brown HT was associated with prevention of ATP formation, resulting in decreased synthesis of DA, NE, and gamma-aminobutyric acid in presynaptic neurons (92). A mild level of growth retardation was found in the males at the 3.0 ~ dietary levels; however, the pigments were found in definite organs, especially in the liver Kupffer cells, at the maximal level diet 3%, along with the tubules of the kidney at both the 1 and 3 levels (93).
Carmoisine (E122, molecular weight 502.431). Carmoisine is a food colorant of the azo dye group which is red to maroon and has an aromatic structure. Carmoisine is usually found in the form of a disodium salt. According to the fact that carmoisine is approved by US Food and Drug Administration (FDA), it is widely utilized in cosmetics, paper, textile, food, pharmaceutical, and agrochemical industries (94). Carmoisine, even at low doses, can modify biochemical markers in important parts of the body. Carmoisine changes the structure of hemoglobin, which leads to the reduction of the helical composition (95). The main metabolite of carmoisine is sulphanilic acid (96). Whenever nitrite compounds exist in several foods combined, they transform into nitrosamines, which are carcinogenic (97). According to the classification of the International Agency for Research on Cancer, azo dyes such as carmoisine are placed in category 3 of carcinogens. Carmoisine can reduce fuel metabolism in the liver (98). In brief, hydrophobic azo dyes are not safe for use due to their ability to induce tumors in the body systems (99).
Brilliant black BN (E 151, molecular weight 867.68). It has been demonstrated that brilliant black BN inhibits CVA6, CVA16, and EV71 strains. A comparison of E151 with the other dyes tested showed the maximal efficacy in the blocking of entry of the virus (100).
BBG (Brilliant blue FCF or E 133). BBG is a water-soluble, functional, and structural analog of FD&C blue dye No. 1. This FDA-approved agent is widely utilized as a coloring agent and food additive which has shown toxicity in humans at doses up to 1g/kg/d in humans (101). Safe doses of BBG may reduce neural injuries or be clinically applicable. In addition, BBG can synthesize proinflammatory agents on defense cells that express P2X7 and influence brain tissue after TBI (102). Carmo et al. (103) mentioned that administration of BBG (45 mg/kg, every 48 h, for 14 days) showed that oral gavage of brilliant blue FCF has statistically significant effects on the survival of the neurons and aggregation of intracellular aβ precursor protein (App) in the hippocampal portion (103). Chronic systemic administration of BBG was associated with cognitive impairment and weakened spatial memory deficit in an AD mouse model. The administration of BBG entirely inversed the inhibitory effects of Ab on the growth and spin genesis of the dendrites. Moreover, this agent might have neuroprotective roles in AD through an independent mechanism of P2X7 based on its chemical properties (104).
Allura Red (E 129, molecular weight 496.42). Allura Red is widely used in candies, ice cream, drinks, and bakery products. According to a previous study, Vorhees reported that the total number of glial cells and neurons after exposure to high doses of Allura Red was decreased by 50 - 60% (105). In contrast, the administration of the low dose of Allura Red did not affect the dendritic length (105). However, the total length of the dendrites in the high-dose Alora red group was reduced by 40% compared to the distilled water group (105). In addition, Noorafshan et al. showed that a high dose of Allura Red leads to several neurological disorders such as multiple sclerosis, brain damage, and ADHD (106). Vorhees et al. demonstrated brain weight loss in mice whose mothers received the red number 40 mg/kg/day for two weeks (105). Likewise, Bawazir showed that the administration of Allura Red 200 mg/kg for eight weeks had adverse effects on the histology of brain tissue (25). Administration of Allura Red was accompanied by a noticeable reduction in the substance of histamine, GABA, and 5-hydroxyindoleacetic acid in all of the examined regions at different times. Also, Allura Red decreased the content of GSH and MDA in the kidney and brain. In the brain, inhibition of endogenous antioxidant defense enzymes is mediated by these free radicals, which then causes brain tissue damage (105). Allura Red caused a decrease in the byazoreductaserats enzymes in the liver cells and into intestinal bacteria through the release of aromatic amines to the organism that caused hyperactivity and distraction in children, while in adults it caused frequent headaches (107-109). Moreover, it has been shown that Allura Red was not carcinogenic in mice (110) or rats (111).
3. Conclusion and Recommendations
Food additive colorants such as azo dyes are largely used in pharmaceutical and food industries but they also may have adverse effects on different body organs. Through various studies reviewed during this study, we found that tartrazine can impair cognition and learning, and damage the prefrontal cortex and cerebellum. Sunset yellow may cause chromosomal aberration, oxidative stress, and prefrontal cortex damage leading to behavioral damage. Ponceau 4R may not affect the brain during fatal growth but previous studies suggested a cholinergic poisoning due to Acetylcholinesterase inhibition may happen upon its use. Metanil yellow produces ROS affecting vital organs including the brain stem, hypothalamus, and stratum decreasing learning ability. Erythrosine B can cause learning disabilities and hyperkinesis in children due to neurotransmitter inhibition or aggregation prevention. Chocolate brown HT may decrease noradrenaline, dopamine, and GABA levels in brain sub-organs, and can leads to mental retardation. Carmoisine was reported as a category three carcinogen, it also may modify biochemical markers even at low doses. Brilliant blue FCF if used in safe doses can prevent neural damage but chronic consumption can cause cognitive impairment and weakened memory. Allura Red can cause a reduction in dendrites’ length, histamine, GABA, and 5-hydroxyindoleacetic acid. Consumers may also develop multiple sclerosis, brain damage, and ADHD.
Azo dyes can increase the quality, color, flavor, and overall preservation of foods yet they may harm the consumer through their metabolism and oxidative stress caused by their metabolism therefore more studies need to look upon the effects of these azo dye additives on different vital organs. We suggest the same review articles to work on the adverse effects of azo dyes on different organs such as the kidney, reproductive system, and gastrointestinal tract. More studies should go over the effects of food additives and the complications of their consumption.