1. Introduction
Lichen planus (LP) is a chronic inflammatory mucosal condition rare in the skin, which is characterized by white plaques and striations or erythematous erosive lesions. About 0.5 - 1% of the world population suffer from the disease (1). Among whom this disease is more common among individuals aged 20 - 49 years old. Lesions can appear anywhere in the oral mucosa; the most common sites are the buccal mucosa, followed by the gingiva and the lateral border of the tongue (2). Despite the potential cause of autoimmunity, oral lichen planus (OLP), with an increased risk for developing squamous cell carcinoma of head and neck (SCCHN), is classified as a premalignant condition by the WHO. In this regard, the incidence of its malignant transformation has varied from 0.07 to 5.3 % in previous studies (3).
The role of autoimmunity in its pathogenesis is supported by many features of OLP, including disease chronicity, adult-onset, female predilection, concurrency with other autoimmune diseases, decreased immune system activity, and the presence of auto cytotoxic T-cell colonies in LP lesions (4). Accordingly, LP patients are more susceptible to other autoimmune diseases, and about 5 - 25% of these patients tend to develop other autoimmune diseases at the same time (5). Several autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), autoimmune thyroiditis, autoimmune hepatitis, ulcerative colitis, alopecia areata, and vitiligo, can also trigger the onset of LP (6-8).
This study reports the complicated OLP management in a 51-year-old woman in post Liver transplant stage who was diagnosed with autoimmune hepatitis and suffered from lupus erythematosus and ulcerative colitis. In this regard, the accompanying symptoms indicated that autoimmune conditions could affect OLP patients simultaneously. A noteworthy point in this study was the existence of three autoimmune diseases with LP, which is not common in the literature. Furthermore, previous studies have focused less on managing patients with a complicated autoimmune condition; however, the present study illustrates a successful model of this case.
2. Case Presentation
A 51-year-old female diagnosed with OLP by biopsy in May 2005 in the Oral Medicine Department of the Tehran University of Medical Sciences returned to this center once more in November 2018 with noticeable complaints of a recurrence of painful oral ulcers on the tongue and other mouth sites after 13 years. She also had been suffering from autoimmune hepatitis over the past five years and had received liver transplantation. By one year after liver transplantation (in June 2013), she had no symptoms, and then the symptoms of ulcerative colitis gradually appeared. Regarding the clinic and para-clinic tests of the patient, systemic lupus erythematous was diagnosed and confirmed almost in 2017 by her physician. Finally, she planned to undergo intestinal surgery in June 2020 because of colon cancer to prevent metastasis after controlling oral lesions.
Her psychological status was acceptable; however, she was nervous about her colon condition and the surgery. Such a feeling was natural, and her great familial support helped her a lot.
The patient received five tablets of prednisolone 5 mg, one allopurinol l100 mg, iron supplementation, and tacrolimus 1 mg three times a day, as well as a monthly remicade injection. According to the patient history, the remicade was injected after 4 or 6, or 8 weeks based on her symptoms, especially the amount of bleeding in colons.
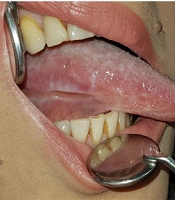
Clinical examination showed a large ulcer (approximately 1 cm in diameter) with a coarse radial semicircular or crescent-shaped white margin and a fibrinoleukocyte membrane in the center, located on the right buccal mucosa at the occlusal line. There was a reticular white lesion on the ventral surface of the tongue, and atrophic lesions were noticed on the dorsal and lateral borders of the tongue, which spread from the middle third to the base of the tongue. In the left buccal mucosa around linea alba, there was a red and white patch/plaque, approximately one centimeter in diameter, next to a small aphthous-like interval ulcer. A homogeneous reticular white plaque was detected in the attached gingiva of the first and second molars (Figure 1). In the extraoral examination, nail deformity was also noticed.
Patient's condition in the first session in November 2018, A, a crescent ulcer located in the middle of right buccal mucosa at the occlusal line with a surrounding white and red plaque and a knife-edge linear ulcer on the right ventro lateral border of the tongue with surrounding radial white plaque.
2.1. Clinical Interventions
We recommended topical corticosteroid as first line of therapy for OLP (9, 10) disease and asked the patient to dissolve the prednisolone tablets in water, gargle the solution, and then swallow it to benefit from the systemic and topical effects of the drug.
The patient had acceptable oral hygiene and followed the instructions. She was under the supervision of a physician for her systemic conditions. Due to ulcerative colitis, she had severe iron deficiency and could not take oral medication; hence, she received injectional iron and vitamin D3 monthly.
We checked her liver and kidney’s functional tests before prescribing drugs for possible systemic side effects, and there was no significant problem. No significant improvement was observed in the two two-week follow-up sessions, so we prescribed dexamethasone 0.5 mg tablets dissolved in 15 cc of chilled boiled water. The patient was asked to gargle it four times a day for 5 minutes, after meals and before going to bed.
Likewise, 25 drops of nystatin 100000 IU suspension was prescribed to be gargled half an hour after corticosteroid mouthwash for 2 - 3 minutes to prevent fungal superinfections.
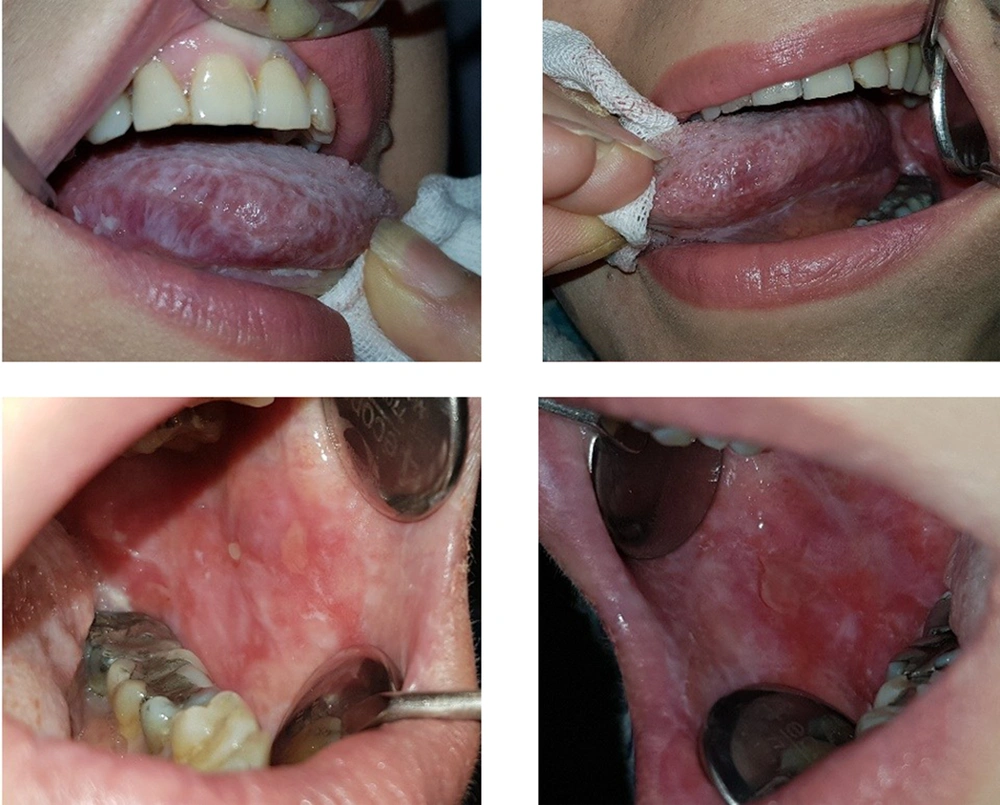
In the third follow-up, with a two-week interval, the healing process began. She was cooperative and followed the recommendations well, the lesions got better, and the ulcers and plaques on the cheeks became smaller; however, she still complained of burning and pain caused by erosions and sores on the tongue and right cheek.
Since the patient's biopsy was old, and the presence of concomitant autoimmune diseases such as lupus erythematosus could possibly cause the resistance of oral lesions to treatment, and on the other hand, drug-related lichenoid lesions could appear similar to LP, after consulting with her physician that assured the negative serological tests for SLE, we eliminate allopurinol from the patient's medicine, which decreased the lesions in the fourth follow up session.
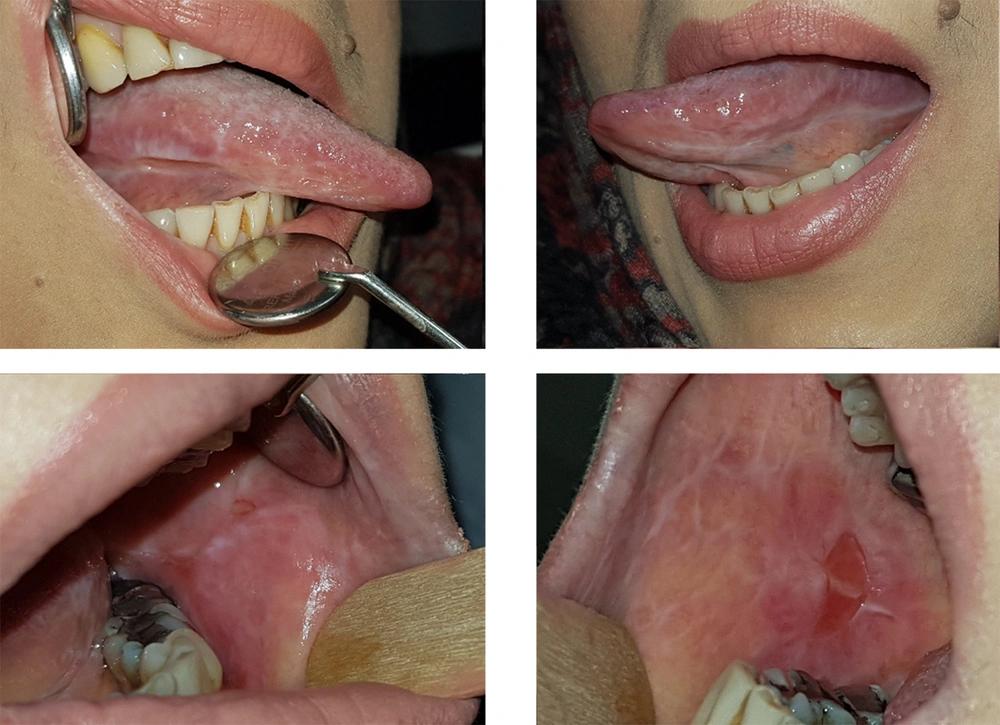
Unfortunately, after a month, near the fifth follow-up session, the recurrence of ulcers and plaques of the mouth and even worse deformity of the nails were observed exactly a few days after injecting Infliximab (Figure 2). According to the patient's history and the potential side effects of infliximab, we consulted with her physician once more and asked him to discontinue the injectable drug for a specific period so that we could replace it with a high dose corticosteroid and then taper the drug After obtaining her physician’s written agreement, the Infliximab injection was discontinued, and we increased the patient's prednisolone dosage by 50 mg.
Moreover, we encouraged the patient to cut down taking sugar, fat, and salt, eat a banana daily to compensate for potassium, calcium, vitamin D3, and Ca, and walk daily to prevent the complications of Cushing's syndrome in high-dose corticosteroids. In the next sessions after 2 - 3 weeks, her blood pressure was measured, and corticosteroid-dependent gastrointestinal considerations such as limiting the use of NSAIDs was noted. Then the healing process was observed, and the oral lesions came under control; hence, we decreased the dosage of corticosteroid by 40 mg and then prednisolone by 35 mg of in the next sessions as long as a small red patch and plaque were observed on the cheeks and the right ventrolateral surface of the tongue.
Finally, we applied cryotherapy to the remaining infected areas on the cheeks and the tongue no to increase the medication dosage, and one month later, the patient was prepared for intestinal surgery with a healthy mouth and a well-controlled oral condition.
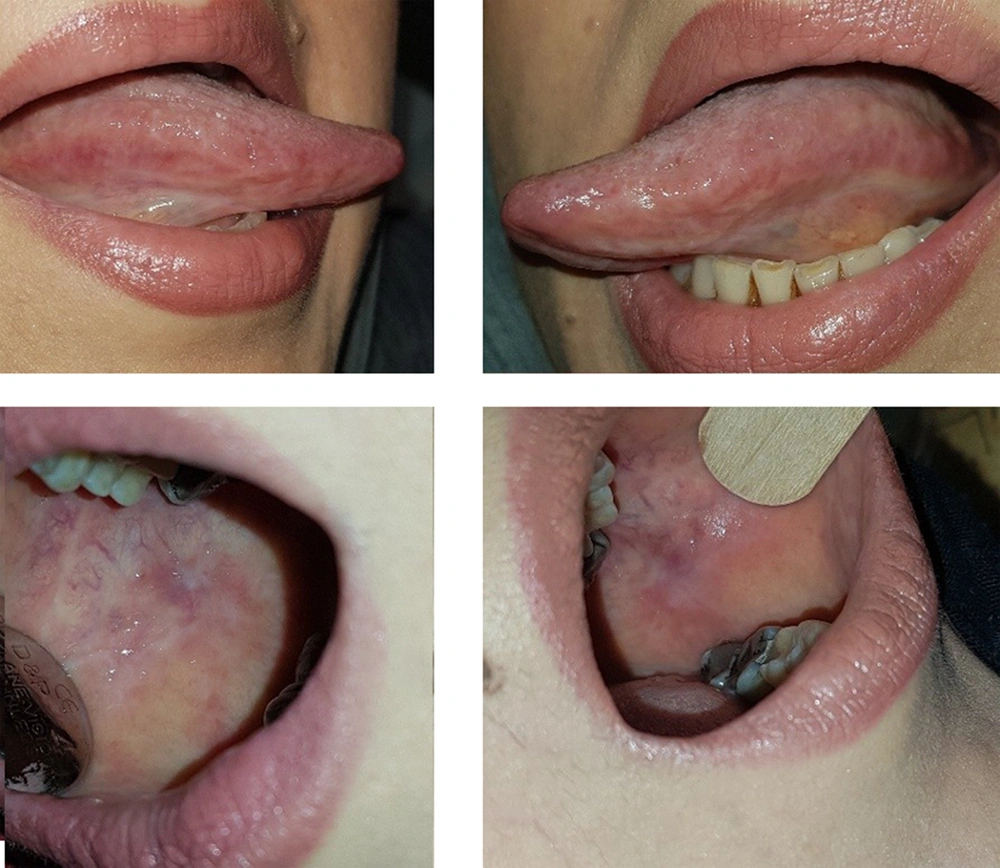
She was followed up after surgery annually via phone calls. Colon surgery was successful. She received 10 mg of prednisolone daily, tacrolimus decreased by 1 mg/day, and no other medication but supplements were taken. Now, she has no oral mucosal complaints (Figure 3).
3. Discussion
Lichen planus (LP) is a chronic inflammatory mucosal disease that rarely affects skin and nails and is characterized by purple polygonal papules, itchy or white plaque-like lesions with or without atrophic or erosive areas. Although the etiology of LP has not been well-understood, previous studies have indicated the autoimmune origin of LP (1). Since patients with an autoimmune disease are prone to other autoimmune diseases, the co-occurrence of LP with other autoimmune diseases is possible (11).
The treatment modalities of LP focus on supportive therapies to facilitate the healing of severe lesions and reduce pain or other discomforts. If there is no pain or discomfort, no intervention is required, and only monitoring the tissue changes may be necessary. However, for patients with more severe symptoms, one or more methods are needed to improve their health status (12).
Corticosteroids can be used directly on the mucous membrane to reduce inflammation associated with OLP. In different forms, such as topical mouthwashes, ointments, or gels, topical corticosteroids continue to be widely used as the first-line treatment for LP. In more symptomatic lesions such as erosive or ulcerative LP, dexamethasone is another potent corticosteroid prescribed as a mouthwash three or four times a day (12).
For those patients not responding well to topical treatments, a systemic form of the drug, such as prednisolone, may be used. If systemic corticosteroids are prescribed, they should be given at the lowest possible dose only for a short period (12). Topical calcineurin inhibitors, including tacrolimus, and topical and oral retinoids are also used to treat these lesions (13).
In a case study with simultaneous vitiligo and LP, triamcinolone acetonide (0.1%) was administered topically 3 - 4 times daily for two weeks, half an hour after meals, for the treatment of OLP lesions. Sargin and Gurer prescribed methylprednisolone (1 mg/kg body weight) and mycophenolate mofetil (1000 mg) for the simultaneous treatment of LP and SLE lesions (14). It should be noted that LE/LP synchronicity is a heterogeneous disease exhibiting the characteristics of both diseases. This overlap may be underestimated because the co-occurrence of LE and LP is uncommon; hence, clinicians need to be more conscious in diagnosing this unusual disease.
Although topical treatment is not sufficient in an overlap of LE/LP, and systemic treatment is required, in Demirci et al.’s case report, the lesions disappeared within two weeks with only tacrolimus 0.1% (9).
The recent case was similar; however, the patient had three simultaneous autoimmune diseases, including SLE, Ulcerative Colitis, and autoimmune hepatitis, which ultimately resulted in liver transplantation with oral ulceration LP lesions.
Since the patient needed bowel surgery, eliminating her oral ulcers was of paramount importance. When the patient was referred, given the patient's previous biopsy, we considered the patient's oral lesions as LP. Like many LP/LE overlap studies, Kiyani and Shahroz used hydrocortisone 400 mg and prednisolone 60 mg at the beginning of treatment, decreased the dose over 18 months to hydrocortisone 200 mg and prednisolone 5 mg, and observed improvement in symptoms. Similarly, we used topical corticosteroids for treatment (15).
In a similar vein, Lospinoso et al. (16) used 50 mg of Astressin, and Patil et al. (17) reported that Methotrexate was successfully used for HIV+ patients with OLP. However, unlike previous articles, we also used the topical effect of a corticosteroid drug used by the patient as a systemic drug (16, 17).
Unlike the aforementioned studies, the recurrence of our patient's lesions did not stop. This was because of several drugs used by the patient for other autoimmune diseases were reported in the literature as a cause of lichenoid reactions. In this study, we suspected drug-related lichenoid reactions (18).
Evidence suggests that TNF-α inhibitors, including infliximab, can stimulate a wide range of inflammatory disease conditions, including skin and mucosal LP-like lesions. Lichenoid reactions may occur within a few weeks after the initiation of therapy or may be delayed for several months. According to our study, at least 13 cases of LP or drug-related lichenoid reactions have been reported in patients treated with anti-TNF-α inhibitors, most of whom were treated with infliximab, etanercept, and adalimumab, and one of whom was with lanercept (18). Several cases of ulcers and mucosal lesions of the skin and mucosa associated with allopurinol have also been reported in the literature (19).
Accordingly, regarding the potential side effects of infliximab, after consulting with the physician, allopurinol and the injectable infliximab were discontinued for a specific period. Then cryotherapy was used for the remaining OLP lesions. The studies have even reported cryotherapy to be more effective than topical corticosteroids (triamcinolone and acetone) (10). The findings were satisfactory, and the healing areas were observed. The patient's management protocols and their validity can be reviewed and re-evaluated in future studies as clinical trial projects.
3.1. Conclusions
Given that autoimmune diseases can co-occur, we successfully managed a patient with recurrent LP in the mouth as oral ulcers in the case of lupus erythematosus, ulcerative colitis, and liver transplantation.
This case, which is not the first case of autoimmune diseases, indicates that managing an oral lesion in the field of other systemic diseases is challenging and that we should be fully aware of the oral manifestations of systemic diseases and their side effects. According to previous studies, the response of oral lesions to different treatments is unique, and on the other hand, the patient's lifestyle and his/her other systemic diseases influence the success or failure of the treatment. This patient's management protocol and its validity can be re-evaluated in future studies as clinical trial projects.