1. Background
Multiple sclerosis (MS) is an autoimmune T-cell-mediated inflammatory disorder involving the central nervous system. It has a variable and unpredictable course with symptoms such as weakness, bladder and bowel incontinence, visual loss, fatigue, cognitive deficits, and mood disorders (1, 2). Statistics suggest that the prevalence of several psychiatric disorders and symptoms increases in MS patients. Anxiety and depression are particularly associated with reduced adherence to treatment, quality of life, and functional status (3).
Anxiety disorders are common problems experienced by MS patients, with a lifetime prevalence of >35% (4). Evidence indicates high levels of anxiety (69%) in MS patients from the onset of the disease (5). Generally, anxiety affects MS patients more frequently than the general population (6). It may be a consequence of neurological and physiological dysfunctions in the course of the disease; in other words, it is a natural reaction to the unpredictable course of this debilitating and chronic condition (7). Additionally, MS patients are prone to depression, fatigue, and low quality of sleep following anxiety disorders. MS-related disabilities, increased fatigue, and MS exacerbations are linked to higher anxiety levels, which may suggest a higher risk of deterioration of anxiety and depressive symptoms over time in patients with multiple sclerosis (pwMS) (6).
Anxiety, as a predictor of depression in the pwMS (7), is associated with increased somatic complaints, social dysfunction, and suicidal ideations (4). This condition is frequently overlooked and undertreated despite its high prevalence and harmful consequences. So far, two categories of treatments, including pharmacological and nonpharmacological, have been proposed for anxiety alleviation. Although pharmacological treatments that effectively reduce anxiety can ameliorate the symptoms, they have numerous side effects. Besides, in many cases, they do not result in the sustained remission of anxiety (8, 9) and do not eliminate internal factors inducing the state of anxiety (10). Therefore, nonpharmacological treatments can be regarded as promising alternative options.
Several meta-analyses have examined and approved the efficacy of psychological interventions for anxiety treatment (11). There is substantial evidence suggesting the benefits of psychotherapy and cognitive behavioral therapy (CBT). However, relapse and residual symptoms are common (12), as only about half of the individuals with anxiety successfully respond to CBT (13). Accordingly, schema therapy (ST) has been developed for chronic and difficult-to-treat patients who are unresponsive to CBT. Schema therapy provides insight, meets emotional needs, and strengthens interpersonal relationships. Generally, it is a rich blend of different treatment models, including CBT, attachment, Gestalt, constructivist, object relations, and psychoanalytic models (14). It may be an alternative treatment for MS patients as it is more flexible than CBT and facilitates coping with a wide range of emotions about MS symptoms and progression, regardless of the disease stage.
According to Young et al., schemas result from five unmet core emotional needs in childhood. The central concepts of ST include needs, early maladaptive schemas, coping styles, and schema modes. Moreover, Young et al. explained that schemas are unconsciously internalized and manifested in specific situations. Another critical component of ST is therapeutic alliance, which plays a role in treatment and is less emphasized in other approaches. Through empathy and awareness, ST can lead to positive changes without inducing a feeling of guilt in individuals and improve their control over life (13).
Several studies have reported that early maladaptive schemas or schema domains are related to the general symptoms of anxiety across psychiatric diseases or in nonclinical samples (14). Nevertheless, there seems to be no research focusing on anxiety-focused treatments for MS (15). According to recent studies, ST can be extended to anxiety and mood disorders (16-18); however, little attention has been paid to the effectiveness of this treatment for MS patients.
2. Objectives
The present study aimed to investigate the effects of ST on anxiety, depression, fatigue, and sleep quality in MS patients.
3. Methods
3.1. Study Design
In this parallel-arm randomized controlled trial, we compared a group of MS patients receiving ST with a group of patients receiving the current local treatment. This research was registered in a registry of clinical trials (http://www.ClinicalTrials.gov, reference No.: NCT04030819) and approved by the Research Committee of Isfahan University of Medical Sciences (approval No.: 396479; ethics code: 30475).
3.2. Participants
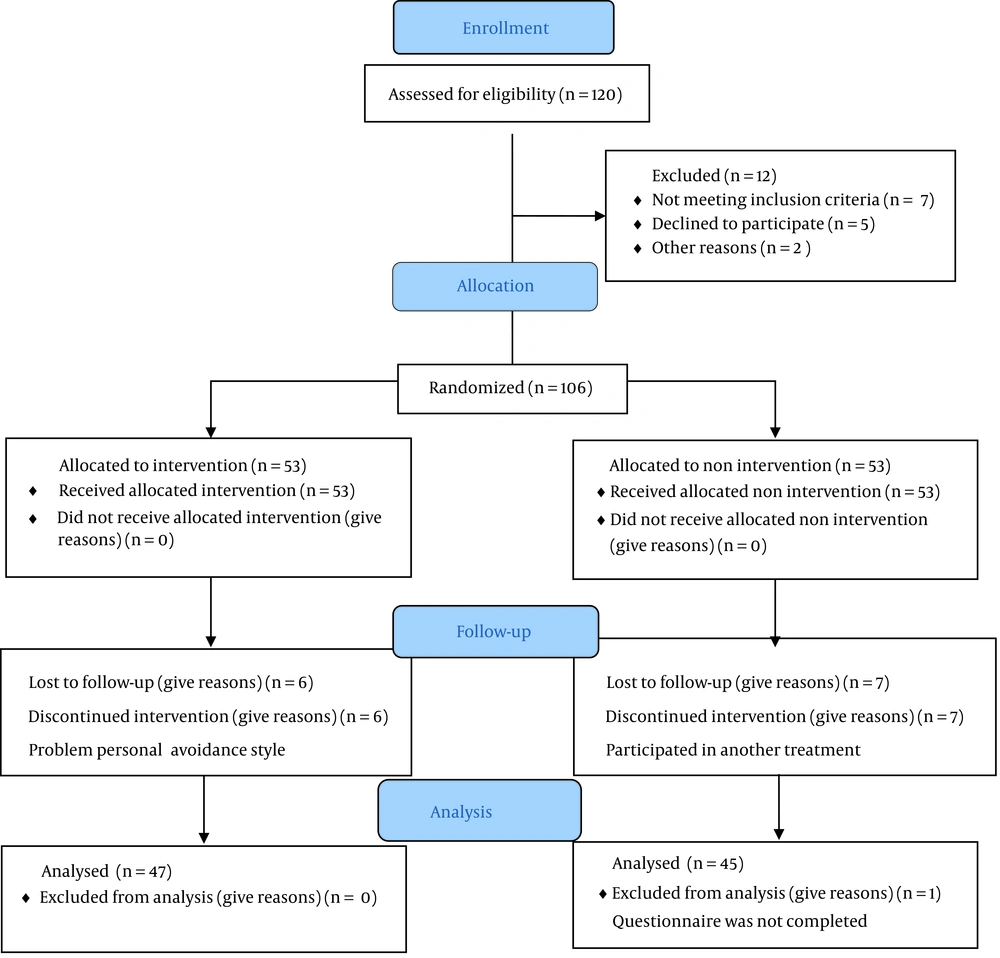
A total of 106 patients with MS were recruited via convenience sampling during 2020 in Isfahan, Iran. The sample size was calculated based on previous studies (19). In this unblinded trial, the research assistant divided the participants into two groups of 53 using the block randomization technique in 27 blocks of 4, which were sealed. After obtaining the consent forms, a neurologist and a psychologist visited all the patients. The inclusion criteria were as follows: Having anxiety based on the Beck Anxiety Inventory (BAI score >26); an Expanded Disability Status Scale (EDSS) score <6; no relapse in the past three months; age above 18 years; informed consent; ability to read and write in Persian; and not being hospitalized. The exclusion criteria were as follows: Cognitive deficits, a history of psychotic disorders, and a history of treatment with disease-modifying drugs or antidepressants in the past three months. Eligible participants were assessed in three intervals: Before the intervention (pretest, Time Point 1), after the intervention (posttest, Time Point 2), and one year later (follow-up, Time Point 3) (Figure 1).
3.3. Intervention
The ST intervention consisted of 20 therapy sessions of 90 minutes (with a 15-minute break) per week, including homework activities. It was developed for a group of six people and delivered by a well-educated psychologist. Although group therapy is not generally structural, we tried to follow the ST clinician's guidelines for group therapy (20). The first session started with welcome remarks, icebreakers, and explanations about ST. The next five sessions focused on schema education and raising schema awareness in everyday life; five sessions concentrated on schema mode management; four sessions focused on cognition and emotions; four sessions concentrated on breaking behavioral patterns; and finally, a farewell and posttest session was held (21).
3.4. Control Conditions
The control group did not receive ST, and their previous treatment continued. They were allowed to withdraw from the study at any time (e.g. if they needed more treatment for anxiety).
3.5. Tools
The primary outcome measure was anxiety, and the secondary outcome measures were depression, quality of life, quality of sleep, and fatigue.
3.5.1. Beck Anxiety Inventory
Anxiety was assessed by the Beck Anxiety Inventory (BAI). Cronbach's α is 0.92, and test-retest reliability is 0.75, as reported by research on BAI (22). Item responses on the BAI range from 0 to 3, where 0 indicates no self-reported symptoms, and 3 indicates more severe symptoms. A total score of 0-7 on the BAI shows minimal anxiety, 8-15 mild anxiety, and 16 moderate or greater levels of anxiety. The Persian version of BAI showed acceptable internal consistency (α = 0.88) and test-retest reliability (r = 0.67) (22).
3.5.2. Beck Depression Inventory
The Beck Depression Inventory (BDI) has 21 multiple-choice questions, each scored on a scale of 0 (not at all) to 3 (severely). Scores range from 0 to 63. BDI-II scores are graded as minimal (0 - 13), mild (14 - 19), moderate (20 - 28), and severe (29 - 63). High internal consistency (Cronbach's alpha: 0.89) and test-retest reliability (r = 0.93) have been mentioned in reports (23). Its Persian version showed high internal consistency (Cronbach's α = 0.87) and reasonable test‐retest reliability (r = 0.74) (24).
3.5.3. The 20-Item Multiple Sclerosis Impact Scale
The 29-item Multiple Sclerosis Impact Scale (MSIS-29) examines physical and psychological scales. Each scale is scored by choosing the answers across the items. The scores range from 0 to 100, where 100 shows the greater impact of the disease on daily functions. All estimations of reliability using Cronbach's alpha were more than the recommended 0.80 (25). Its Persian version showed test-retest reliability (intra-class correlation coefficients > 0.70), high internal consistency (Cronbach's alpha > 0.70), and good validity (26).
3.5.4. The Pittsburgh Sleep Quality Index
The Pittsburg Sleep Quality Index (PSQI) is a self-report questionnaire with 19 items, scored on a 0–3 scale. Its score can be calculated by totaling the scores of the seven components, and the total score ranges from 0 to 21. Lower scores show a healthier sleep quality. A global PSQI score >5 results in a diagnostic specificity of 86.5% and sensitivity of 89.6% (kappa = 0.75, P < 0.001) in differentiating good and poor sleepers (27). The Persian version of PSQI has a good content validity index (≥ 0.78) with a Cronbach's alpha of 0.65 (28).
3.5.5. The Fatigue Severity Scale
The Fatigue Severity Scale (FSS) has nine items that assess the fatigue motor aspects. Its main emphasis is on assessing the severity of fatigue symptoms and their impact on people's daily functioning. Each item is scored from 1 to 7 on a Likert scale. The cut‐off score for fatigue was set to ≥4. The analysis reported internal consistency and reliability for FSI (Cronbach α = 0.93). Stable values were obtained over time (2.94 ± 0.90 vs. 2.90 ± 0.74; P = 0.27 in test-retest variability (29). In the Persian version, the ICC, 0.93 for the total score, 0.88 for VAS, and Cronbach's alpha of 0.96 were calculated. The VAS and the total score showed a significant correlation with the 36-Item Short Form Survey (SF-36) vitality subscale (r = -0.73 and r = -0.69, respectively) (30).
3.5.6. The Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is used to diagnose and evaluate anxiety and depression in nonpsychiatric patients (31). The scores are as follows: 0 – 7: Normal or no anxiety, 8 – 10: Mild anxiety, 11 – 14: Moderate anxiety, and 12 – 21: Severe anxiety. Its Persian version is admissible to patients (99%). Cronbach's alpha was 0.86 for the HADS depression subscale and 0.78 for the HADS anxiety subscale (32).
3.5.7. Statistical Analysis
Descriptive statistics were reported as mean (standard deviation [SD]) for continuous variables and frequency (percentage) of patients for categorical variables. A Kolmogorov-Smirnov test was performed to analyze the data distribution. Repeated-measures analysis of variance (ANOVA) was performed for each measure (MSIS, PSQI, BAI, HA, HD, BDI, HADS, FSS, EDSS) considering the between-subject factor "group" (intervention and control) and the within-subject factor "time" (Pre (pretest), Post (posttest), and F (follow-up). The results of repeated-measure analyses were recorded as eta square (Eta) and P-value. The level of significance was set to 0.05 (2-tailed). All the statistical analyses were performed in IBM SPSS Statistics v. 18 (IBM Corporation, Armonk, NY, USA).
4. Results
The values of the mean (SD) of age in the intervention and control groups were 36.55 (7.36) and 35.91 (7.76), respectively. The values of the mean (SD) of EDSS in the intervention and control groups were 1.31 (0.90) and 1.29 (0.87), respectively. The results of the independent t-test showed that the mean values of age (P = 0.663) and EDSS (P = 0.913) did not differ between these two groups (Table 1).
| Variables | Intervention (N = 53) | Control (N = 53) | Total | P-Value | |||
|---|---|---|---|---|---|---|---|
| Frequency | Relative Frequency | Frequency | Relative Frequency | Frequency | Relative Frequency | ||
| Age, y | 0.150 | ||||||
| Less than 30 | 6 | 31.6 | 13 | 68.4 | 19 | 17.9 | |
| 30 to 40 | 29 | 50.9 | 28 | 49.1 | 57 | 53.8 | |
| Higher than 40 | 18 | 60.0 | 12 | 40.0 | 30 | 28.3 | |
| Education level | 0.714 | ||||||
| High school diploma and lower | 5 | 50 | 5 | 50 | 10 | 9.4 | |
| Bachelor's | 38 | 48.1 | 41 | 51.9 | 79 | 74.5 | |
| Master's | 9 | 56.3 | 7 | 43.8 | 16 | 15.1 | |
| Doctor of philosophy and higher | 1 | 100 | 0 | 0 | 1 | 0.9 | |
| Sex | 0.819 | ||||||
| Female | 41 | 50.6 | 40 | 49.4 | 81 | 76.4 | |
| Male | 12 | 48.0 | 13 | 52.0 | 25 | 23.6 | |
| Marital status | 0.800 | ||||||
| Married | 43 | 49.4 | 44 | 50.6 | 87 | 82.1 | |
| Single | 10 | 52.6 | 9 | 47.4 | 19 | 17.9 | |
The Frequency Distribution of the Participants Based on Their Demographic Characteristics
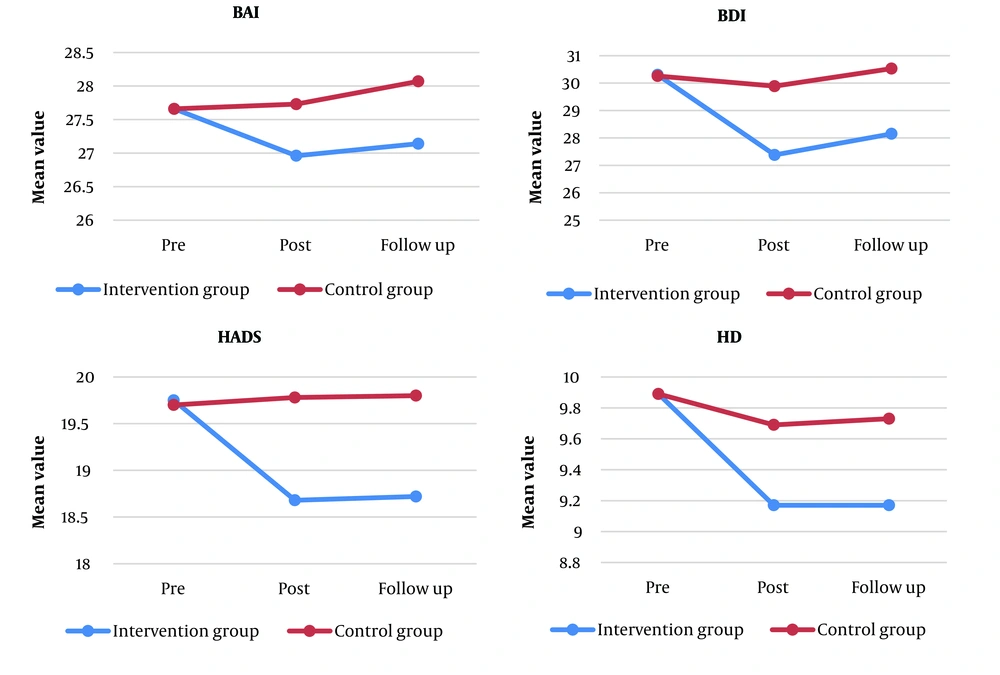
Table 2 reports the mean and SD values of the studied variables between the two groups at different times. The t-test showed significant differences between the mean values of all the variables of the two groups at pre-intervention (P > 0.414). The mean values of the variables of BAI, BDI, HADS, HD, and HA were significantly different between the two groups at post-intervention and follow-up (P < 0.024). The results revealed significant differences between subjects in HA (P < 0.001), HD (P = 0.003), BDI (P = 0.047), and HADS (P < 0.001.) Moreover, the within-subject result showed that BAI (P < 0.001), HA (P < 0.001), HD (P < 0.001), BDI (P < 0.001), and HADS (P = 0.001) had significant changes over time.
| Variables | Intervention (N = 53) | Control (N = 53) | P-Value |
|---|---|---|---|
| BAI | |||
| Pre | 27.66 ± 1.39 | 27.66 ± 1.53 | 0.999 |
| Post | 26.96 ± 1.49 | 27.73 ± 1.75 | 0.024 |
| F | 27.14 ± 1.47 | 28.07 ± 1.60 | 0.005 |
| P-value | > 0.001 | > 0.001 | - |
| BDI | |||
| Pre | 30.30 ± 4.67 | 30.26 ± 3.26 | 0.962 |
| Post | 27.38 ± 4.40 | 29.89 ± 3.67 | 0.004 |
| F | 28.15 ± 4.12 | 30.53 ± 3.33 | 0.003 |
| P-value | > 0.001 | 0.003 ± | - |
| HADS | |||
| Pre | 19.75 ± 1.07 | 19.70 ± 0.82 | 0.761 |
| Post | 18.68 ± 0.98 | 19.78 ± 1.20 | < 0.001 |
| F | 18.72 ± 1.10 | 19.80 ± 1.08 | < 0.001 |
| P-value | > 0.001 | 0.564 | - |
| HD | |||
| Pre | 9.89 ± 0.87 | 9.89 ± 0.64 | 0.999 |
| Post | 9.17 ± 0.70 | 9.69 ± 0.60 | < 0.001 |
| F | 9.17 ± 0.84 | 9.73 ± 0.58 | < 0.001 |
| P-value | > 0.001 | 0.318 | - |
| HA | |||
| Pre | 9.85 ± 0.72 | 9.81 ± 0.52 | 0.757 |
| Post | 9.26 ± 0.49 | 9.82 ± 0.65 | < 0.001 |
| F | 9.51 ± 0.69 | 10.11 ± 0.86 | < 0.001 |
| P-value | > 0.001 | 0.022 | - |
| FSS | |||
| Pre | 42.96 ± 3.42 | 42.81 ± 2.48 | 0.795 |
| Post | 42.43 ± 3.44 | 42.91 ± 3.44 | 0.500 |
| F | 42.57 ± 3.73 | 43.07 ± 3.77 | 0.531 |
| P-value | 0.613 | 0.770 | - |
| MSIS | |||
| Pre | 71.32 ± 6.62 | 71.66 ± 8.59 | 0.820 |
| Post | 70.34 ± 6.55 | 70.80 ± 8.23 | 0.767 |
| F | 70.36 ± 6.57 | 70.82 ± 8.25 | 0.767 |
| P-value | 0.187 | 0.786 | - |
| PSQI | |||
| Pre | 9.06 ± 1.13 | 8.89 ± 0.99 | 0.414 |
| Post | 8.98 ± 0.97 | 8.66 ± 0.68 | 0.073 |
| F | 9.00 ± 1.000 | 8.70 ± 0.67 | 0.099 |
| P-value | 0.931 | 0.743 | - |
Mean and Standard Deviation Values of the Studied Variables Between the Two Groups at Different Times a
Figure 2 depicts the changes in the mean values of the studied variables in the two groups at different time intervals.
Table 3 illustrates the effectiveness of the intervention on the studied variables. The group effect was significant for BDI, HADS, HD, and HA variables (P < 0.047). The highest values of the eta coefficient regarding the group effect were related to HADS (0.155) and HA (0.129), respectively. The time and group × time interaction effects were also significant for BAI, BDI, HADS, HD, and HA (P < 0.001). The greatest values of eat at the time effect were related to the variables of BDI (0.251) and HD (0.155), respectively; at the group × time interaction effect, the greatest values of eta were assigned to the variables of BDI (0.238) and HADS (0.150), respectively. None of the effects were significant for the variables of FSS, MSIS, and PSQI. The effect size of Cohen's d disclosed that the intervention effect was significant in reducing the values of BAI, BDI, HADS, HD, and HA (d > 0.5).
| Variables | Intervention (N = 53), Mean | Control (N = 53), Mean | Cohen's d | Between Subjects (Group) | Within Subjects | ||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Time × Group | ||||||||
| Eta | P-Value | Eta | P-Value | Eta | P-Value | ||||
| BAI | 0.038 | 0.063 | 0.100 | <0.001 | 0.135 | <0.001 | |||
| Pre-intervention | 27.66 | 27.66 | 0 | ||||||
| Post intervention | 26.96 | 27.73 | -0.597 | ||||||
| Follow up | 27.14 | 28.07 | -0.595 | ||||||
| BDI | 0.037 | 0.047 | 0.251 | <0.001 | 0.238 | <0.001 | |||
| Pre-intervention | 30.30 | 30.26 | 0.009 | ||||||
| Post-intervention | 27.38 | 29.89 | -0.617 | ||||||
| Follow up | 28.15 | 30.53 | -0.634 | ||||||
| HADS | 0.155 | <0.001 | 0.092 | < 0.001 | 0.150 | <0.001 | |||
| Pre-intervention | 19.75 | 19.70 | -0.179 | ||||||
| Post-intervention | 18.68 | 19.78 | -0.764 | ||||||
| Follow up | 18.72 | 19.80 | -0.614 | ||||||
| HD | 0.092 | 0.003 | 0.155 | <0.001 | 0.078 | 0.001 | |||
| Pre-intervention | 9.89 | 9.89 | 0 | ||||||
| Post-intervention | 9.17 | 9.69 | -0.795 | ||||||
| Follow up | 9.17 | 9.73 | -0.775 | ||||||
| HA | 0.129 | <0.001 | 0.092 | <0.001 | 0.099 | <0.001 | |||
| Pre-intervention | 9.85 | 9.81 | 0.060 | ||||||
| Post-intervention | 9.26 | 9.82 | -0.989 | ||||||
| Follow up | 9.51 | 10.11 | -0.773 | ||||||
| FSS | 0.004 | 0.561 | 0.004 | 0.576 | 0.003 | 0.655 | |||
| Pre-intervention | 42.96 | 42.81 | 0.050 | ||||||
| Post-intervention | 42.43 | 42.91 | -0.129 | ||||||
| Follow up | 42.57 | 43.07 | -0.131 | ||||||
| MSIS | 0.001 | 0.812 | 0.010 | 0.351 | 0.017 | 0.220 | |||
| Pre-intervention | 71.32 | 71.66 | -0.044 | ||||||
| Post-intervention | 70.34 | 70.80 | -0.061 | ||||||
| Follow up | 70.36 | 70.82 | -0.061 | ||||||
| PSQI | 0.034 | 0.081 | 0.002 | 0.797 | 0.001 | 0.955 | |||
| Pre-intervention - | 9.06 | 8.89 | 0.159 | ||||||
| Post-intervention | 8.98 | 8.66 | 0.380 | ||||||
| Follow up | 9.00 | 8.70 | 0.345 | ||||||
Effectiveness of the Intervention on the Studied Variables Based on the Data of the Two Groups at Different Times
5. Discussion
The present study investigated the effects of ST on anxiety in MS patients. The results indicated the significant effects of this therapy on anxiety and depression and the modification of some schemas. According to ST, anxiety is developed if people fail to meet their needs, do not gain the approval of their loved ones, and experience feelings of guilt (33). Studies on the impact of ST on anxiety have reported the efficacy of this treatment (14, 34, 35), although few relevant studies have been conducted on MS patients.
Recent research suggests that ST can reduce anxiety in the pwMS (36). In ST, patients learn not to suppress their emotions, balance a one-sided relationship (by reducing other people's responsibilities and paying increased attention to their own needs), and become cognizant of their own emotions and feelings of loneliness and helplessness, which are considered the main sources of anxiety. Additionally, overcoming subjugation and self-sacrifice, modifying the locus of control, and improving interpersonal relationships should be considered in reducing anxiety. Note that there are differences between patient and nonpatient populations.
In relapsing-remitting MS patients, the unpredictable course and relapse of MS are associated with stress as the patients have no knowledge of the timing, severity, or consequences of relapse (37). This disability is directly related to the patients' uncertainty over future events, driving them toward chronic fear or fear of disease progression, as well as anxiety, by reducing their chance to be prepared for the future (38). Therefore, pwMS experience fear and anxiety simultaneously and experience anxiety at different cognitive and emotional levels. It is known that ST changes the individuals' roles by explaining the reasons for behaviors, events, and emotions besides cognitive development. According to existentialism, although patients cannot remove the source of their anxiety, they can control it; therefore, awareness of the cause of behavior can reduce anxiety and fear of death. The feelings of power and control associated with awareness are also important (39).
Schema therapy had no significant effect on fatigue, quality of life, and sleep quality. MS is generally associated with several problems, such as difficulty coping with MS diagnosis, understanding the disease and its process, and dealing with the so-called "hidden" symptoms, such as sexual dysfunction (prevalence of 40 - 80% in women and 50 - 90% in men) (40), pain (50 - 86%) (41), and bladder dysfunction (75%) (42). Besides, the quality of sleep and fatigue may be affected by multiple factors, including age, sex, physiological, hormonal, pharmacological, and naturalistic processes (43), brain volume, metabolism (38), and seasonal changes in sleep duration (44). Overall, a combination of factors, including sleep, fatigue, and quality of life, can be introduced as the main reasons for the nonsignificant effects of ST.
5.1. Limitations and Suggestions
This study had some strengths and limitations. According to the criteria proposed by Ingram, Hayes, and Scott (45), stability and acceptability were the main strengths of this research. Alleviation of anxiety and depression was maintained for six months. Additionally, a 10% attrition rate revealed that the patients were interested in the ST treatment.
On the other hand, this study was limited by a lack of safety due to the associated family conflicts and nonacceptance of individual changes by others. In individuals with medical conditions, it is often difficult to clearly differentiate psychological symptoms from physical ones. There is also little research on the efficacy of ST for chronic diseases. Besides, during follow-ups, intervening factors could have adverse effects on the validity of long-term outcomes. Moreover, there were some variables affecting the mental health of MS patients in Iran, such as social, economic, and political restrictions (44). Finally, the patients faced disease-related challenges, such as difficulty procuring MS medicines. Therefore, further studies focusing on important MS symptoms, such as pain, bladder dysfunction, and sexual disorders, are suggested in other cultural groups.
5.2. Conclusions
The present study revealed that ST could effectively alleviate anxiety and depression in the PwMS. Nonetheless, this treatment did not significantly affect the FSS, MSIS, or PSQI variables. It is recommended that future studies investigate the effects of complementary therapies on MS patients.