1. Context
Acrylamide (AA) is a toxic compound that forms in the thermal processing of food products. Before its recognition in food, as a contaminant applied, this industrial chemical produced glues, plastic, paper, and treatment of wastewater and drinking water (1, 2). This heat process contaminant is made in food heating processes such as frying, baking, toasting, roasting, and baking, especially in starchy food like potatoes (3, 4). Also, foods that were boiled or nonthermally treated are free from AA, and protein-rich foods have a low content of this contaminant. AA levels in some processed foods are shown in Table 1. The existence of AA in food products for the first time was reported by Swedish National Food Administration in April 2002 (5). After that, many investigations confirmed the occurrence of this toxic compound, particularly in carbohydrate-rich food, which was hated above 120°C (6). International Agency for Research on Cancer (IARC) has classified the AA at grope 2A. It is a carcinogenic ingredient probably carcinogenic to humans. The investigation has demonstrated AA can have carcinogenic properties in animals and humans. The monomeric form of AA is a neurotoxic compound for humans, which can cause tumors in many organs (5).
| Food | Acrylamide (µg/kg = ppb) |
|---|---|
| Almonds, roasted | 260 |
| Baked products: Bagels, breads, cakes, cookies, pretzels | 70 - 430 |
| Biscuits, crackers | 30 - 3200 |
| Cereals, breakfast | 30 - 1346 |
| Coffee powder | 170 - 351 |
| Corn chips, crisps | 34 - 416 |
| Crispbread | 800 - 1200 |
| Fish products | 30 - 39 |
| Gingerbread | 90 - 1660 |
| Meat and poultry products | 30 - 64 |
| Nuts and nut butter | 64 - 457 |
| Peanuts, coated | 140 |
| Potato, boiled | 48 |
| Potato chips, crisps | 170 - 3700 |
| Potato, French-fried | 200 - 12000 |
| Potato, puffs, deep-fried | 1270 |
| Snacks, other than potato | 30 - 1915 |
| Soybeans, roasted | 25 |
| Sunflower seeds, roasted | 66 |
| Taco shells, cooked | 559 |
Regarding chemically, AA is a colorless, odorless, water-soluble, and polymerizable compound used in textile and paper industries. The maximum formation of AA in foods is at a temperature of 160°C until 180°C in the presence of asparagine amino acid and reducing sugars like glucose (6). Various investigations were done by the food and drug administration (FDA) and other organizations, indicating that the level of AA in potato chips and other products containing fried potatoes, bakery products, and coffee is higher than in other foods. The FDA and WHO stated that AA's daily intake in developed countries is 0.3 - 0.8 µg/kg bw, while its amount in fried potato products is reported to be 400 - 1500 µg/kg (3, 7).
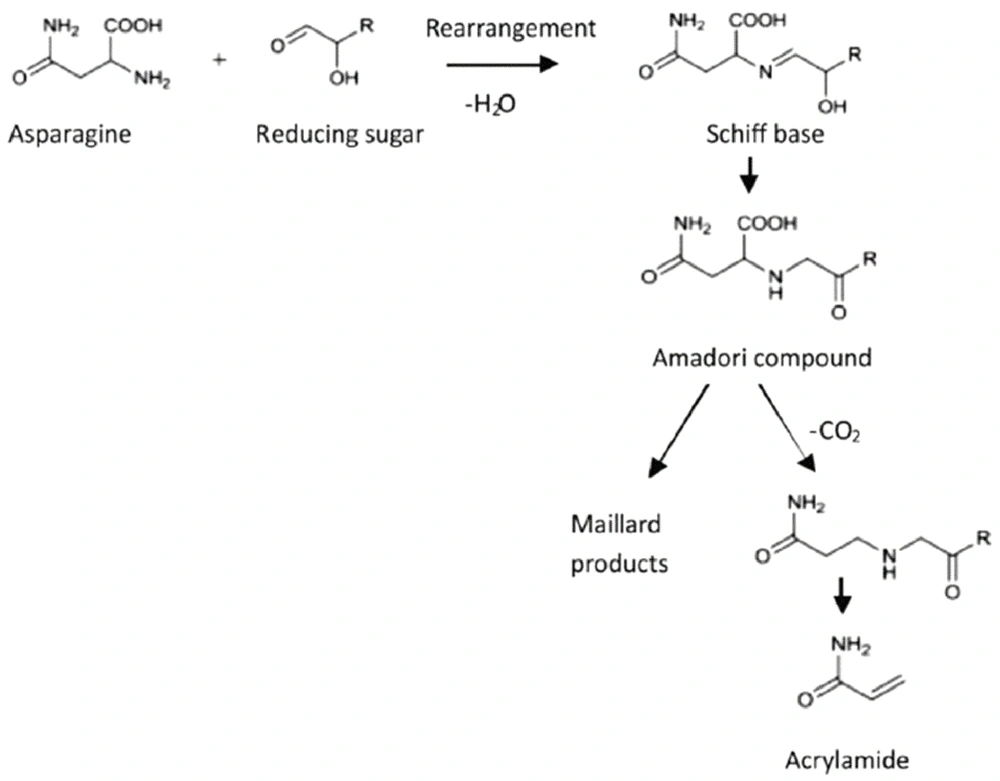
Additionally, AA or 2-propenamides are compounds with properties like odorless, white crystalline solid at room temperature and have high solubility in water. These compounds are created during the Millard reaction in wild foods. In this reaction, the carbonyl group of reducing sugars reacts with the amino group of amino acids, especially free asparagine. It creates a Schiff base intermediate which leads to AA formation during decarboxylation in the following steps (8). Due to carcinogenicity, cell mutation, male fertility defects, weakening of the body's immune system and the creation of nervous disorders by this chemical pollutant, which negative effect of its have been proven not only in laboratory animals but also in humans and on the other hands with the increasing development of fried potato products production industries and high consumption of these products it is necessary to implement different condition such as optimizing agriculture soil and planting verities with low AA precursors (reducing sugars and asparagine), the temperature and suitable duration of storage of potato , inhibition of precursor by enzymes, optimization the frying process in term of temperature and time of processing, choosing the right type of oil for frying, blanching of potato slices, treatment with organic acids, mineral salts, amino acids and lactic fermentation of potato slices before processing and using antioxidants, these procedures provided optimal processing conditions to reduce the amount of this chemical pollutant in fried potato products by maintaining the quality, taste, color and nutritional value (3, 9). In addition to this, AA is also formed through Acrolein. In general, the formation of AA is from the reaction with ammonia. Factors such as differences in food composition, process parameters, and final cooking conditions can change the amount of AA in the final products. In this review, we presented the necessary procedures reduction of AA until 2022.
2. Formation of AA
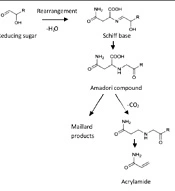
The prime hypotheses for forming acrylamide focused on frying lipids, vegetable oils, and baking starchy foods. The essential precursors for developing AA include: Acrolein, acrylic acid 3-amino propionamide, Schiff base, and decarboxylated Amadori product (6, 10-12). According to studies, the Millard reaction is the main pathway to forming AA in food products. This reaction can occur between the asparagine amino acid and reducing sugar like glucose during the heating processes of food and create this heat-contaminated substance (13). Millard reaction is a complex sequence reaction that can occur during the thermal processing of food products. During this nonenzymatic reaction, glucose, and fructose, as reducing sugar. Condense with amino acids, mainly asparagine, to yield N-glycoside. Melanoidin is produced through various steps. Finally, decarboxylation of the Schiff base makes the AA (Figure 1) (6, 14, 15). Low moisture content and high temperature can increase the formation of AA. Another pathway for the construction of AA is the acrolein pathway. Mentation compound is created in oils when glycerol degrades and forms Acrolein above their smoking point. Then Acrolein oxidized to acrylic acid and produced acrylamide. Asparagine provides the amino group in this reaction, and acryl acid provides the carbon source (16, 17).
Different factors and methods are effective in reducing the formation of AA in fried potato products.
Two stages before and during processing can investigate factors affecting the reduction of AA formation in fried potato products.
3. Influential Factors and Methods Before Processing
practical factors and methods before processing include genetic modification to produce potato varieties with less or without reducing sugars or free asparagine amino acid. Studies have shown that if the agricultural soil contains less sulfur, the amount of asparagine amino acid in the potato produced from this soil is low, making less AA after processing. Also, if the agricultural soil contains more nitrogen, the free amino acids, and protein level increases, and more AA is created in the potato products during processing (7).
Sucrose, glucose, fructose, and starch are potatoes' sugars. At temperatures above 10°C, sugars and starch are in equilibrium. At temperatures below 10°C reducing sugars begin to increase, and these sugars can participate in the Millard reaction and the formation of AA. It investigated the storage of Japanese potato samples for 18 weeks at 2,6,8,10 and 18°C. The amount of reducing sugars and AA was significantly increased at a temperature lower than 8°C, but there was no change in the amount of asparagine during storage. In Swedish potato samples, in addition to reducing sugars, the amount of asparagine also increased during storage in cold conditions. In potatoes, storage should ensure that the storage temperature does not cause the formation of reducing sugars. The potato can be placed again at room temperature by creating such a state to reduce the number of decreasing sugars (18). It adds 140 mmol/kg of 1-glutamine, 2-arginine, 3-lysine, 4-glycine, and 5-asparagine amino acids to the asparagine/glucose system. Processing at 180°C for 25 min showed that glutamine 70%, arginine 50%, lysine 88%, and glycine 91% decreased the formation of AA, while adding asparagine increased the formation of AA about two times.
In a study on potato chips, the pretreatment process of potatoes with amino acids shows that two amino acids, including glycine and lysine, had the most significant effect on reducing the formation of AA. Adding 35 and 240 mmol/kg of fructose to the glucose/asparagine system caused the amount of AA to increase by 4 and 3 times, respectively, while adding the same amount of glucose caused an increase of about 1.6 and 4 times in the formation of AA. The reason why the shape of AA decreases in a high concentration of sugars has not yet been determined. The role of fructose in forming AA is twice that of glucose in potatoes. Studies have shown that potatoes with a content of 1 g of reducing sugar per kilogram produce more than 500 µ/kg of AA when fried, and to reduce this amount, the level of reducing sugars should be less than 1 g/kg (18).
Blanching potato chips with hot water (1 - 8 min at a temperature of 95°C) leads to a logarithmic decrease in the amount of reducing sugars, total sugars, and glucose, which this reduction reducing sugar is 51 - 62% of the initial amount. Also, the amount of AA formation in the fried-shallow process is about four times higher than in the blanching and deep-fried process (at 190°C). Soaking potatoes in the acetic acid solution for 60 min at 20°C and then frying them led to a 90% reduction in AA content caused by releasing free amino acids and reducing sugars during treatment. Soaking potato in a solution of 10 g/L of citric acid and sodium tetra pyrophosphate for 80 min at 50°C decreases the processing temperature from 190°C to 150°C (19).
This action leads to the reduction of AA by releasing the free amino acid and reducing sugar. The role of citric acid in preventing the formation of AA is more significant than sodium tetra pyrophosphate. Acid acetic is more effective than citric acid. Soaking potato slices in 1% sodium hydroxyl solution and frying them in an oven temperature of 105°C for 20 - 30 min can reduce AA formation.
Soaking potato slices in 1% NaCl solution significantly reduces the formation of AA, and treatment with CaCl2 solution prevents the formation of AA up to 95% during frying. Immersing potato slices for 60 min before frying in solutions of 0.001, 0.01, and 1 molar vanadyl sulfate inhibited the formation of AA by 30.3%, 53.3%, and 89.3%, respectively. This study showed that the binding of vanadium ions to asparagine and the reduced pH of potato samples considerably caused the absence of AA formation in fried potato products. Lactic fermentation of potato chips by Lactobacillus Plantarum NC8 at 37°C for 45 and 120 min can reduce AA by 48% and 71%, respectively, after deep frying at 170°C. If the blanching operation is performed before fermentation after deep frying, the amount of AA will decrease by 79 and 94 percent at two fermentation times of 45 and 120 min, respectively (20).
The reason for this phenomenon is the usage of reducing sugars by Lactobacillus plantarum and the production of lactic acid, which leads to a decrease in pH from 5.7 to 4; in this case, the amount of glucose decreases from 8.610 to 7.9 mg/100 mL during 3 hours of fermentation, but there is no change in the amount of asparagine amino acid (21-23).
It is preheating potato slices with a microwave (10, 20, and 30 seconds at 850 w) and deep frying at a temperature of 150, 170, and 190°C, respectively. It reduces by 36, 41, and 60% in AA.
In this investigation, the surface and core of potato chips measured the amount of AA. To be much higher than the heart-found amount on the cover. The microwave process reduced the frying time, improved water transfer from depth to the surface, and finally decreased AA formation on the products' surface (24). Since the AA is formed from asparagine amino acid and reducing sugars through the Millard reaction, removing these precursors with L-asparaginase enzyme in raw materials prevents from formation of AA in the final product. It used L-asparaginase enzyme solution in a simulated potato matrix depending on the conditions (enzyme dose, incubation time, and temperature), resulting in a reduction of 50 - 90% in the formation of AA. Adding asparaginase enzyme to potato led to 96% hydrolysis of asparagine amino acid into aspartic acid. As a result, AA formation was reduced during cooking and frying.
Glucose can be oxidized by using glucose oxidase enzyme, which can prevent participation in AA production. The effect of enzymes confirms that inhibiting the precursors of AA (asparagine amino acid and reducing sugars) can reduce its formation in food (25, 26). Soaking the potato slice in a solution of 2% to 5% (w/w) eight hydrocolloids for one hour showed that alginic acid at a concentration of 5% reduces the amount of AA in fried potato by about 30%. Its reduction was insignificant at a concentration of 2%; if the soaking time reached 5 hours, the drop ranged from 30 to 60% (27).
Treatment of potato slices before frying with polyphenolic antioxidants of cranberry and oregano and treatment with chickpea was done in a study. Treatment with chick paste caused found a further reduction in the formation of AA due to the improving effect of chicken paste protein on potato amino acid. The low effectiveness of antioxidants shows that the construction of AA is done in a non-oxidative system. In another study, the addition of spice flavonoids caused a significant decrease in the formation of AA in potato chips. The results of a new study indicated that antioxidants could not effectively destroy AA or stop or even stimulate its appearance. Still, their oxidative products can destroy AA and its precursor (asparagine), and as a result, the formation of AA can be prevented (28).
4. Influential Factors and Methods During Processing
The results of studies show that at the temperature of 120°C, the amount of formation of AA is meager, and the minimum temperature for formation should be above 120°C. Deep frying of potato products at a temperature of 140 - 160°C increases AA formation linearly. By increasing the temperature, the amount of construction of AA is increased. But at a temperature higher than 170°C, AA formation depends on the processing time; the shorter the processing time, the less amount of AA is formed during the process. Frying of potatoes under the atmospheric condition at a temperature of 165°C for 4 min led to the formation of 521 µg/kg of AA in rose-withe potatoes, 649 µg/kg in Atlantic potatoes, and 466 µg/kg at Shepody's potatoes has been changed. While frying under vacuum conditions at a temperature of 118°C has led to a 94% reduction in acrylamide formation in potato chips compared to the atmospheric method.
The amount of acrylamide formation in potato chips that have been blanched and surface fried at a temperature of 180°C is about four times more than deep frying at a temperature of 190°C. surface frying of potatoes without blanching at 180°C increase the amount of AA formation (9, 29, 30).
Although oxidation of edible oils during frying can lead to forming AA precursor, its appearance cannot be attributed to the hydrolysis and oxidation of edible oils. Studies have shown that the oxidation of lipids during the frying of potatoes does not lead to a significant increase in the formation of AA; among the oils, palm oil leads to the formation of more AA during frying. Also, trying potato slices in 20 samples of virgin olive oil at a temperature of 180°C for 5, 10, and 15 min has shown that the amount of AA formed decreased, and this decrease was more in 5 min. Also, this decrease was attributed to the phenolic compounds present in the oil. Among this virgin olive oil, the sample with high ortho-diphenyl caused a more significant reduction in the amount of AA (29-31).
The study investigated the effect of 15 vitamins on the amount of AA formation in the model system (asparagine and glucose) in fried potato products. Fat-soluble vitamins are ineffective in reducing AA, and among the water-soluble vitamins, B2, B5, and PL can decrease the amount of AA formation in the model system. In potato products, vitamin C, B3, and PN caused the reduction of AA 11, 50, and 35%, respectively (32).
Water plays a complex role in the formation or removal of AA. At low humidity, the activation energy for the construction of AA in potatoes is more than its value for the Millard reaction. As a result, the amount of AA formation decreases. In another study, at higher temperatures and water activity (aw), the amount of AA formation is higher. Millard reaction is mainly done in medium water activity. At very high humidity, the formation of AA and the browning response are reduced (18).
We are reducing the internal pH of potato by 28% fumaric acid, 29% tartaric acid, and 25% phosphoric acid from 5.72 to 2.96, resulting in a 70% reduction in the amount of AA. And pH 6 increases the formation of AA, and again from pH 8 to 10, the amount of AA formation decreases. When the pH goes from 8 to 4, the rate of AA formation in potato chips at 160°C decreases by about ten times (18, 33).
A study investigated the effect of turmeric extract on the AA content of fried potato chips. Blanched Potato slices were immersed in turmeric extract 0, 10, 15 w/w for 15 min and then fried in oil. Moisture level, oil absorption, shrinkage, color, AA content, and sensory characteristics were measured and compared with the control sample. The obtained results showed that with the increase in the concentration of the extract, the moisture percentage of samples increased, and the fat content and shrinkage decreased. Also, by increasing the engagement of turmeric extract, potatoes' brightness and redness decreased, and potatoes' yellowness increased. Increasing the concentration of turmeric up to 10% caused a 47% decrease in the amount of AA compared to the control sample. Except for the taste, the sensory characteristic improved with the concentration of turmeric extract. The overall results showed the use of 10% turmeric extract.
Along improvement of the qualitative and sensory properties, it led to a significant reduction in the formation of AA. Fried potatoes can be suggested as a method to reduce the appearance of this toxic compound. The decline of AA by immersing potato samples in turmeric extract with a concentration of 10% can be attributed to the presence of curcumin in turmeric extract, which has potent antioxidant activity and acts as a preservative against oxidation (34, 35). Another study reported a similar trend regarding the reduction of AA formation and an oil absorption of potato slices treated with olive oil extract during frying (36).
Fat oxidation is the most critical pathway of AA formation in oil-rich foods such as fried potatoes. During deep frying of oils rich in linoleic and linolenic acids, Acrolein is formed significantly, which can lead to the formation of AA during oxidation and reaction with ammonia. On the other hand, methyl linoleate, a non-oxidized lipid in the presence of glucose, can be oxidized to unsaturated carbonyl compounds by free radicals and involved in the carbonyl-asparagine reaction and production of AA. By inhibiting free radicals while turning into phenoxyl radicals, polyphenolic compounds are considered chain-breaking antioxidant compounds and vent the oxidation of fats. Inhibiting the oxidation of fats and then reducing the formation of AA has been the result of investigating phenolic compounds and the effectiveness of this effect related to the concentration of polyphenolic compounds and their structure. As it is said, the development of ortho diphenols is much more than monophenols (37-39).
In addition, blanching pretreatment of potato pieces at a temperature of 85°C, as well as immersing potato pieces in turmeric extract solution, the content of reducing sugars and asparagine decreased in potato slices before frying, which was another influential factor in reducing the amount of AA in fried potatoes (40).
The role of hydrocolloids in reducing AA can be related to different dimensions. Any factor (pH, water activity, temperature, frying time, and food formulation) that can affect the Millard reaction can affect the amount the AA formation (41). Immersion of potato slices in an acidic solution creates conditions in which the amine group of asparagine prevents its reaction by reducing the sugar by taking protons and creating anions (42, 43). Also, hydrocolloids minimize the formation of AA despite their ability to retain water and increase humidity.in addition to reducing pH and increasing humidity, we can mention the antioxidant property of the hydrocolloids. Antioxidants affect the Millard reaction so that they can affect AA formation (44). Polysaccharide compounds of soya soluble in water have antioxidant properties, and it has been proven that this substance can prevent the oxidation of oil and other foods prone to oxidation; despite this property, it prevents the oxidation of lipids and thus precludes the AA formation (44, 45). The apparent difference between soybean water-soluble polysaccharide and Arabic gum in reducing AA. Due to the unique properties of this polysaccharide, such as its high adhesive strength. In other words, the property of film formation and good adhesion of soluble polysaccharides of soya in water has caused it to be in contact with the thin sheet of potato as much as possible. It affects the acidity of the environment and the reduction of moisture loss. By forming a uniform coating on its surface, it reduces the absorption of fat and the formation of compounds resulting from oxidation. It also prevents the development of nonenzymatic browning of Millard by establishing bonds with water molecules and reducing pH.
Sadat Mousavian et al. reported carboxymethyl cellulose and Keira hydrocolloids in a concentration of 0.3 to 0.7. It was different combinations of oil to minimize AA in fried potatoes in the form of slices observed that hydrocolloids can significantly reduce the formation of AA in chips, and the best treatment in their research was able to minimize AA by 62.9% (41). Zeng et al. reported that using pectin, alginic acid, and xanthan in the chemical model and potato slices can reduce AA. Also, increasing the immersion time in the case of all three types of hydrocolloids could significantly reduce the amount of AA formation. The effect was much higher than the concentration of hydrocolloid solution, which agrees with the result of this research. The reduction of AA by the hydrocolloids is a process dependent on concentration and immersion time, so that with the increase of both, the amount of AA reduction changes dramatically (27). Choosing the right oil is one of the most critical factors that affect the formation of AA in fried potato products. According to some research, unsaturated fatty acids and lecithin in sesame oil reduce the amount of oil absorption. By creating a crust on the surface of the food, they prevent AA formation (46). Due to potent antioxidants and unique lignin compounds, sesame oil has higher thermal stability than other vegetable oils (47). The results clearly showed that the mechanism of AA reduction in the samples was in two ways. First, the antioxidant compounds in the sesame can block the chain reactions auto-oxidation of fats, free radicals, and chelate metals effective in the oxidation of fats. It stops the oxidation process of Acrolein and, as a result, reduces the formation of AA. These compounds react with nitrite compounds, block them, and prevent them from participating in the processes leading to forming AA or effective intermediate compounds (48).
5. Conclusions
Due to the increasing industrial production of fried potato products , people great interest in consuming them, the high probability of forming AA during thermal processes, carcinogenicity, creation of cell mutation and neurological disorders of AA and by considering that fried potato products are the most crucial source of this chemical pollutant ,it is possible to choose varieties of potatoes that have a lower amount of reducing sugar, storing at a temperature above 8°C, frying potatoes under vacuum and at a temperature lower than 120°C, using deep frying instead of surface frying, avoiding pH in the range of 6 - 8, pre-process or soaking potato slice in amino acids such as glycine and lysine, blanching of potato slices and soaking them in acetic acid, use of asparaginase and glucose oxidase to minimize AA precursors, soaking in NaCl and CaCl2 salt solutions, pre-process or treatment with pectin hydrocolloids and alginic acid, vitamins, lactic fermentation before processing, use of antioxidants compounds during the process, or by using two or more of the mentioned methods, it is possible to minimize the amount of AA in fried potato products.