1. Context
The 2005 Nobel Prize in Medicine and Physiology was jointly awarded to Barry J. Marshall and J. Robin Warren for introducing Helicobacter pylori (H. pylori) and its function in gastritis and peptic ulcer disease. This gram-negative pathogen is capable of colonizing the stomach of more than half of people worldwide (1), with a higher prevalence in less industrialized countries (2).
In addition to extra gastric gastrointestinal malignancies such as stomach ulcers and gastritis (3), its role in gastric cancer has been determined (4). The International Agency for Research on Cancer (1994) identified H. pylori as a human-class carcinogen because of its association with gastric cancer (5). Gastrointestinal cancers such as gallbladder, anal, pancreatic, esophageal, colon, small intestine, gastric, colorectal, and liver cancers are among the deadliest cancers in the human population (6). While the role of H. pylori in the development of several cancers, such as pancreatic cancer (7), adenocarcinomas (4) gastric carcinoma (8) has been well established, there is a growing interest in its relationship with adverse pregnancy outcomes (9) and gynecological complications (10).
Several meta-analyses have demonstrated a significant association between H. pylori infection and birth defect (9), fetal growth restriction (9), gestational diabetes mellitus (9, 11), spontaneous abortion (9), preeclampsia (9), hyperemesis gravidarum (12) and infertility (10). The association between H. pylori infection and respiratory system disorders has been a focus of study for several years. Recent sparsely-published meta-analyses have demonstrated a significant increase in the risk of lung cancer (13) and asthma (14) in patients with H. pylori infection.
Studies in various fields of medicine are being published exponentially, which means the processing of new information in mass volume by clinicians. Due to the lack of consistent repeatability, findings from individual studies are often not valid enough to provide confident answers. The statistical method of meta-analysis can combine the results of different studies on a subject and resolve the conflict between researchers (15).
This overview aims to produce knowledge on this fascinating topic about H. pylori-associated diseases and cancers using published previous meta-analyses. Naturally, when the care providers have a comprehensive knowledge of the disease, the quality of their care will be improved.
2. Search Strategy
The search strategy was to identify relevant meta-analyses on the valid MEDLINE, Scopus, and CENTRA databases b two separate reviewers from the inception until 2022. The main keywords in English included “Helicobacter pylori” OR “H. pylori” OR “Helicobacter species”) AND (“Esophageal Cancer “OR “Esophageal Neoplasms” OR “pancreatic cancer” OR “pancreatic carcinoma” OR abortion OR miscarriage OR pre-mature OR preterm OR postmature OR stillbirth OR Outcomes OR intrauterine death OR fetal death OR low birth weight infant OR small for gestational age infant OR SGA OR macrosomia OR Intrauterine growth restriction OR fetal growth restriction OR IUGR OR birth defect OR hydatid mole OR hydatidiform mole OR ectopic pregnancy eclampsia OR eclmpsi OR preeclampsia OR preeclampsia OR gestational diabetes OR GDM or asthma OR lung cancer) AND (meta-analysis). In addition, other references from related review articles in these databases were also searched and reviewed. The search was also conducted at seminars, conferences, sed congresses to find related articles.
2.1. Selection Process
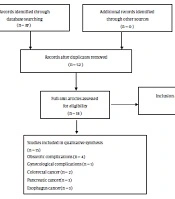
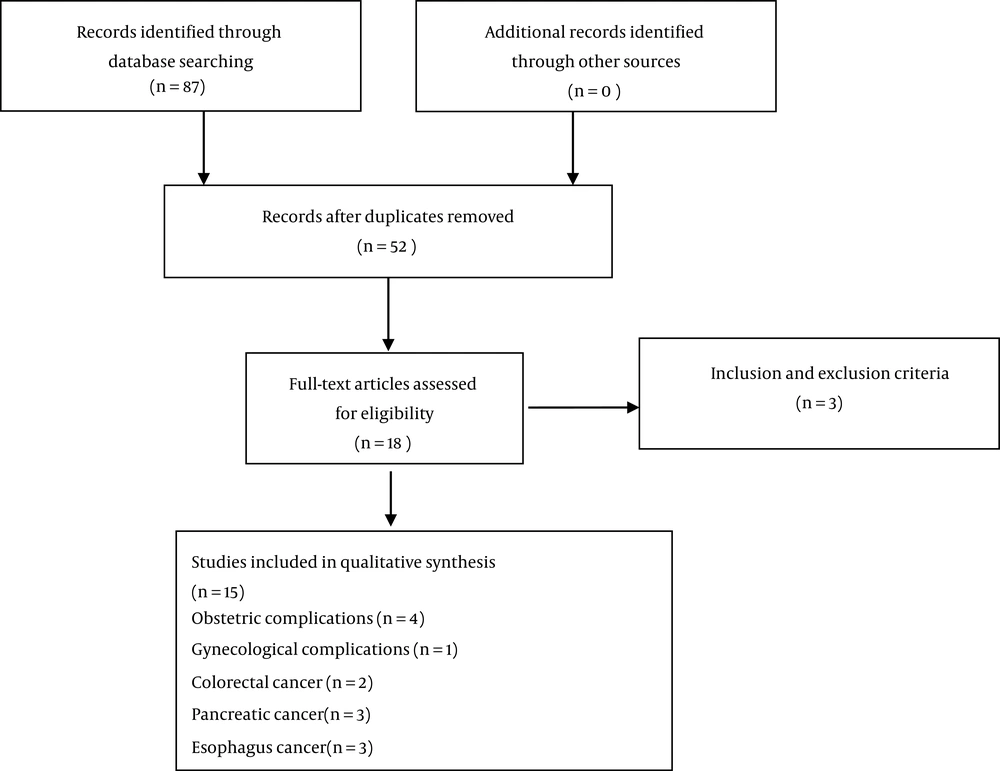
Duplicates were removed through screening titles and abstracts. The full text of all remaining articles was reviewed for eligibility assessment. In the case of finding the study’s abstract related to the title of the present study, the full text of the article was requested through correspondence with the corresponding author. Only published articles in English were searched to control the biases of search strategies (Figure 1).
2.2. Eligibility Assessment
Inclusion criteria were published meta-analyzes of case-control or cohort or cross-sectional studies that reported the association between H. pylori infection and at least one of the adverse pregnancy outcomes in pregnancy, infertility, colorectal cancer, pancreatic cancer, esophagus cancer, and asthma. Exclusion criteria were cell culture studies, animal studies, the literature type of letter to the editor, abstract, original article, and review articles. Non-English articles were excluded.
2.3. Data Extraction
The following data were extracted from included studies by a predesigned form developed by the research team: authors, year of publication, sample size, type of study, outcomes, main result, and design. Two researchers retrieved the study contents. Any disagreement between the reviews was resolved by a third reviewer (Table 1).
| Authors and Year of Publication | Sample Size | Study Design | Outcomes | Main Results |
|---|---|---|---|---|
| Schulte et al. (16) | Ten studies | Case-control study | Pancreatic cancer | A significant association between H. pylori and pancreatic cancer (OR = 1.13; 95 % CI; 0.86 - 1.50) |
| Putra and Putra (13) | Nine studies | Case-control, cohort, and cross-sectional | Lung cancer | A significant increase in the risk of lung cancer in patients with positive H. pylori infection (P = 0.0002) |
| Gao et al. (17) | 35 studies (345,886 patients) | Case–control study | Esophageal squamous cell carcinomas | No significant correlation between esophageal squamous cell carcinomas and H. pylori infection (OR: 0.84; 95% CI: 0.64 - 1.09/OR: 0.74; 95% CI: 0.54 - 0.97) |
| Wang et al. (18) | Five studies (770 cases and 785 control) | Case-control study | Risk of asthma | H. pylori infection had a significant association with the risk of asthma (OR = 1.01; 95% CI = 0.82 - 1.24) |
| Xiao et al. (19) | Twenty-seven studies | Case-control study | Esophageal carcinoma | A significant association between H. pylori seropositivity and development of pancreatic cancer (OR; 1.47 and 1.22 - 1.77) |
| Trikudanathan et al. (7) | Six studies (2,335 patients) | Case-control, cohort, and cross-sectional | Pancreatic cancer | A significant association between H. pylori and pancreatic cancer (adjusted odds ratio [AOR] = 1.38, 95% CI: 1.08 - 1.75; P = 0.009) |
| Islami and Kamangar (20) | 19 studies | Case-control or cohort studies | Esophageal squamous cell carcinomas | No significant relationships between esophageal squamous cell carcinomas and H. pylori infection (OR = 1.10; 95% CI: 0.78 - 1.55; I2 = 73%) |
| Afsar et al. (21) | Six studies (1274 participants) | Observational studies | Micronutrient deficiencies | H. pylori infection was not significantly higher among pregnant women with micronutrient deficiencies than those without (OR = 1.12, 95% CI 0.88 to 1.42, P = 0.37). |
| Li et al. (10) | Seven studies (1,902 patients) | Case-control study | Infertility | H. pylori infection was significantly associated with infertility (OR = 1.45, 95% CI: 1.197 - 2.160; I2 = 36.5%, Z = 3.15, P = 0.002) |
| Zhan et al. (9) | Thirty‐one studies (22 845 participants) | Cross‐sectional study, case‐control study, or cohort study designs | Spontaneous abortion, preeclampsia, gestational diabetes mellitus, fetal growth restriction, and birth defect | A significant association between H. pylori infection and spontaneous abortion (OR: 1.50; P = 0.024), preeclampsia (OR: 2.51; P < 0.001), gestational diabetes mellitus (OR: 2.03; P < 0.001), birth defect (OR: 1.63; P = 0.03), fetal growth restriction (OR: 2.28; P = 0.01) |
| Ng et al. (12) | 38 studies (10 289 patients) | Cross-sectional and case-control studies | Hyperemesis gravidarum | A significant association between H. pylori infection and hyperemesis gravidarum (OR = 1.348, P < 0.001) |
| Tang et al. (11) | The 31 studies (5852 H. pylori infection positive and 8196 H. pylori infection) | Observational studies | Spontaneous fetal growth restriction, hyperemesis gravidarum, preeclampsia, gestational diabetes mellitus | No significant association between H. pylori infection and spontaneous the onset of labor (OR, 1.00; P = 0.98) and a significant association between H. pylori infection and fetal growth restriction (OR, 1.45; P < 0.001), hyperemesis gravidarum (OR, 14.45; P < 0.001) preeclampsia (OR, 2.68; P < 0.001) and gestational diabetes mellitus (OR, 2.63; P < 0.001) |
| Rokkas et al. (22) | 28 studies | Case–control, cross-sectional, cohort | Colon neoplasia, colon cancer, colon polyps | A significant relationship between Helicobacter pylori infection and colon neoplasia (pooled = 1.41; 95% confidence interval: 1.24 – 1.60; P = 0.000). colon cancer (OR = 1.3; 95% confidence interval: 1.07 – 1.59; P = 0.01) and colon polyps (OR = 1.5; 95% confidence interval: 1.26 – 1.79; P = 0.000) |
| Wang et al. (23) | Twenty-seven (3450 adenocarcinoma patients, 1304 adenomatous polyp patients, and more than 4000 controls)” | Case-control studies | Relationship between Helicobacter and colorectal adenocarcinoma and adenomatous | Helicobacter pylori was associated with an increased risk of colorectal adenocarcinoma and adenomatous polyp (odds ratio (OR): 1.24, 95%CI: 1.12 – 1.37, P = 0.66; OR: 1.87, 95%CI: 1.53 – 2.28, P = 0.81) |
| Wu et al. (5) | Twenty-seven studies (3792 cases of colorectal adenoma and 3488 cases of colorectal cancer) | Case-controlled studies | Relationship between Helicobacter and colorectal adenoma | H. pylori infection was associated with an increased risk of colorectal adenoma (CRA) (OR = 1.66; 95% CI 1.39 1.97; heterogeneity P = 0.008) |
2.4. Methodological Assessment of Meta-analyses
The Measurement Tool Assess Reviewer (AMSTAR) approach was used to report the quality of each included systematic review in this overview. There are 12 questions on this checklist which are listed in Table 2, and bias using ratings of “yes,” “partial yes,” or “no.” AMSTAR demonstrated good face and content validity (24). Two reviewers independently evaluated the methodological quality of the meta-analyses (Table 2).
| Questions | Studies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (18) | Afsar et al. (21) | Wu et al. (5) | Zhan et al. (9) | Tang et al. (11) | Ng et al. (12) | Rokkas et al. (22) | Li et al. (10) | Islami and Kamangar (20) | Trikudanathan et al. (7) | Gao et al. (17) | Xiao et al. (19) | Schulte et al. (16) | Putra and Putra (13) | Wang et al. (23) | |
| 6 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 items | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | No |
| 3 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 4 items | No | Yes | No | No | Yes | No | No | No | No | No | No | No | No | Yes | No |
| 5 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 items | Yes | Yes | No | No | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 9 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 11 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12 items | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
a (1) Address a focused question (2) Comprehensive literature search on database research (3) The systematic search and reproducibility (4) Publication bias (5) Clearly-defined inclusion and exclusion criteria (6) Was the methodological quality of each study assessed using predetermined quality criteria? (7) Description of key features of studies (8) The meta-analysis was conducted correctly (9) Similarity of the results from study to study (10) Is the effect size practical and relevant? (11) The estimate of the effect (12) Organization of results?
3. Results
We included recently published high-quality meta-analyses and assessed the relationship between H. pylori infection and gynecological or obstetric complications (Table 1).
3.1. Helicobacter pylori Infection and Birth Defect and Fetal Growth Restriction
A meta-analysis with 22 845 participants showed a significant association between H. pylori infection and birth defect (P = 0.03) (9).
Two meta-analyses showed a significant association between H. pylori infection and fetal growth restriction, as showed in Zhan et al. met-analysis (OR: 2.28; P = 0.0195% CI: 1.21 - 4.32) (9) and in the meta-analysis by Tang et al. (OR, 1.45; 95% CI, 1.26 - 1.66, P < 0.001) (11).
3.2. Helicobacter pylori Infection and Obstetric Complications
Two meta-analyses showed a significant association between H. pylori infection and gestational diabetes mellitus, namely Zhan et al. meta-analysis (OR = 2.03; P < 0.001) (9) and Tang et al. met-analysis (OR, 2.63; 95% CI, 1.51 - 4.59, P < 0.001) (11). Two meta-analyses assessed the relationship between spontaneous rate and H. pylori. One meta-analysis showed a significant association between H. pylori infection and spontaneous abortion (OR: 1.50; 95% CI: 1.05 - 2.14; P = 0.024) (9). However, another meta-analysis showed that H. pylori infection was not significantly correlated with the spontaneous abortion rate (OR, 1.00; P = 0.98) (11).
Two meta-analyses showed a significant association between H. pylori infection and preeclampsia, namely the Zhan et al. met-analysis (OR: 2.51; P < 0.001; 95% CI: 1.88 - 3.34) (9) and Tang et al. met-analysis (OR, 2.68; 95% CI, 2.02 - 3.56, P < 0.001) (11). Three meta-analyses showed a significant association between H. pylori infection and hyperemesis gravidarum during pregnancy, namely Ng et al. meta-analysis (OR = 1.348 (95% CI: 1.156 - 1.539, P < 0.001) (12), Tang et al. meta-analysis (OR, 14.45; 95% CI, 10.24 - 20.38, P < 0.001) (11), and Zhan et al. met-analysis (OR: 2.51; 95% CI: 1.88 - 3.34; P < 0.001) (9).
In a meta-analysis of 6 studies (with 1274 participants: 553 cases and 721 controls), no significant association was observed between H. pylori infection and micronutrient deficiencies (OR = 1.12, 95% CI: 0.88 to 1.42, P = 0.37). In the subgroup analysis, no significant correlation was observed between H. pylori infection and vitamin B12 (P = 0.22), folate (P = 0.73), and ferritin (P = 0.4) deficiencies. However, iron-deficiency anemia (IDA) was positively correlated with H. pylori infection (P < 0.0001) during pregnancy (21).
3.3. Association Between Helicobacter pylori Infection and Gynecological Complications
Regarding the relationship between H. pylori infection, a meta-analysis of Seven studies involving 1 902 patients concluded H. pylori infection was significantly associated with infertility (OR = 1.45, 95% CI: 1.197 - 2.160; I2 = 36.5%, Z = 3.15, P = 0.002) (10).
3.4. Helicobacter pylori Infection and Colorectal Cancer
Regarding the relationship between H. pylori infection and colorectal cancer, in the first meta-analysis, twenty-seven studies, including 3792 cases of colorectal adenoma (CRA) and 3488 cases of colorectal cancer (CRC), were identified. Overall, H. pylori infection was associated with an increased risk of CRA (OR = 1.66, 95% CI 1.39 - 1.97, I2 = 54.3%) and CRC (OR = 1.39, 95% CI 1.18 - 1.64, I2 = 35.8%) (5). In the second meta-analysis, twenty-seven case-controlled studies were included. Helicobacter pylori was associated with an increased risk of colorectal adenocarcinoma and adenomatous polyp [odds ratio (OR) 1.24, 95% CI 1.12 – 1.37, P = 0.66; OR 1.87, 95% CI 1.53 – 2.28, P = 0.81] (23).
3.5. H. pylori Infection and Pancreatic Cancer
Regarding the relationship between H. pylori infection and pancreatic cancer, two meta-analyses showed a significant association between H. pylori seropositivity and the development of pancreatic cancer (adjusted odds ratio [AOR] = 1.38, 95% CI: 1.08 - 1.75; P = 0.009; six studies;2,335indivuals) in Trikudanathan et al. meta–analysis (7) and in Xiao et al. meta–analysis (OR; 1.47 and 1.22 - 1.77) (19). In contrast to the above meta-analyses, more recent ones did not confirm this association as showed in Schulte et al. meta–analysis (OR = 1.13; 95 % CI; 0.86 - 1.50) (16).
3.6. Helicobacter pylori Infection and Esophagus Cancer
Regarding the relationship between H. pylori infection and esophageal adenocarcinoma (EAC), H. pylori were associated with significantly reduced risks of EAC as showed in Rokkas et al. meta-analysis (OR = 0.52; 95% CI, 0.37 – 0.73; P < 0.001) (4), Islami and Kamangar meta-analysis (OR = 0.56 0.46 - 0.68; I 2 = 15%) (20); however, there were no significant relationships between esophageal squamous cell carcinomas (ESCC) and H. pylori infection as showed in Rokkas et al. (4) met-analysis (pooled OR = 0.85; 95% CI, 0.55 – 1.33; P = 0.48), in Islami met-analysis (OR = 1.10; 95% CI: 0.78 - 1.55 (I 2 = 73%) (20), and in Gao et al. met-analysis (OR: 0.84; 95% CI: 0.64 - 1.09/OR: 0.74; 95% CI: 0.54 - 0.97) (17).
3.7. The Association Between Helicobacter pylori Infection and the Risk of Asthma and Lung Cancer
One meta-analysis included four cross-sectional and five cohort investigations. A significant increase in lung cancer risk was observed in patients with positive H. pylori infection (I2 = 50%; P = 0.0002) (13). A second meta-analysis included five case-control investigations with 785 controls and 770 cases. Based on overall data, H. pylori infection had a significant association with the risk of asthma (OR = 1.01; 95% CI = 0.82 - 1.24) (18).
4. Discussion
The H. pylori infections were significantly associated with general adverse pregnancy outcomes such as gestational diabetes mellitus, preeclampsia, and hyperemesis gravidarum and adverse birth outcomes such as birth defect and fetal growth restriction. H. pylori infection was not associated with a deficiency of micronutrients (B2, folate, and ferritin) but was associated with an increased risk of iron-deficiency anemia during pregnancy. The results also indicated a significant association between H. pylori infection and an elevated risk of colorectal cancer, colorectal adenoma, and asthma. Moreover, H. pylori infection is significantly associated with adverse pregnancy outcomes such as lung cancer. The results of the metanalyses were not consistently reproducible regarding spontaneous abortion.
Approximately 80% of pregnant women experience nausea and vomiting (25). Hyperemesis gravidarum (HG) refers to recurrent severe nausea and vomiting, which cause insufficient food intake and is associated with dehydration and ketoacidosis during pregnancy (26). There is still no complete understanding of the pathogenesis of HG. Some known factors in this field are related to the central nervous, hormonal, psychological, immune, placental, and digestive systems (27). The preferred curative approach is to prescribe triple treatment, containing a proton pump inhibitor and two antibiotics, metronidazole and amoxicillin, for two weeks. However, caution should be taken in drug interventions during pregnancy because of teratogenicity risks. Metronidazole and amoxicillin are not recognized as teratogenic antibiotics (12). In addition, proton pump inhibitors do not represent a major teratogenic risk in humans (28). Two meta-analyses evaluated the association between H. pylori (Hp) infection and HG (12, 29). They stated that Hp infection was also associated with congenital malformations, gestational diabetes, fetal growth retardation, spontaneous miscarriage, and preeclampsia (9).
According to Ahmed et al., HG symptoms can be significantly improved through Hp eradication in infected pregnant women. Screening should be appended to HG examinations, in particular, if it is prolonged or resistant to traditional treatment. Modified, high-dose, non-teratogenic dual therapy for Hp eradication can relieve HG in incurable cases with minimal complications (30). However, no specific clinical guidelines have been issued to date on the eradication of Hp infection during pregnancy (12)
The possible underlying pathogenesis for H. pylori to induce PE can be explained in several ways. Firstly, the oxidative damage caused by the free radicals may increase superoxide anion and hydrogen peroxide and lead to greater lipid peroxidation.
This condition leads to endothelial damage and causes high blood pressure (31). secondly, H. pylori may damage the vessels by acting as a trigger mechanism for clotting cascade or
activated lymphocytes to form and secrete cytokines, such as tumor necrosis factor. Thirdly, pylori infection from Cag-A strains could modulate IL-18 release (32). One meta-analysis concluded that H. pylori infection was significantly associated with infertility (10). These associations can be explained via several mechanisms. High anti-H. pylori antibody in cervical mucus may interfere with spermatozoa capacitation and motility and play a role in infertility (33). Treatment of seminal H. pylori significantly increased sperm motility in infertile asthenozoospermic men (34). PCOs are among the most common reasons for infertility. A possible association was observed between H. pylori seropositivity and PCOs (35, 36). Anti-helicobacter antibodies reacted with human spermatozoa's tails and the pericentriolar area (37). A meta-analysis reported a significant association between H. pylori infection and birth defects (9). An Updated Comprehensive meta-analysis published in 2022 showed that H. pylori-positive patients had lower serum vitamin B12 and folate levels than H. pylori-negative patients (38). These micronutrients may greatly influence fetal nervous system development during pregnancy, and vitamin B12 (cobalamin) supplementation has also been associated with a reduced risk of congenital malformations (39). Folic acid prevents birth defects (40). Two meta-analyses showed a significant association between H. pylori infection and gestational diabetes mellitus, one being Zhan et al. meta-analysis (OR = 2.03; P < 0.001) (9) and another being Tang et al. meta-analysis (OR, 2.63; 95% CI, 1.51 - 4.59, P < 0.001) (11). The association between H. pylori infection and insulin resistance can be explained via several biological mechanisms. First, changes in glucose metabolism might lead to chemical alterations in the gastric mucosa that can dramatically increase H. pylori infection (41). A second explanation is an increase in the proinflammatory cytokine levels in response to H. pylori gastric infection, resulting in structural alterations to the insulin receptors inhibiting their interaction with insulin (42).
A risk of CRA, CRC (5), colorectal adenocarcinoma, and adenomatous polyp (23) were observed in patients with positive H. pylori infection. There is still no complete information on how H. pylori infection increases the colon cancer risk. However, some possible mechanisms for this carcinogenesis are impaired cell cycle and inflammation. H. pylori infection carries a pathogenicity island, the cytotoxin-associated gene A (CagA) protein. CagA presents positive H. pylori infection and can lead to a greater risk of gastric cancer. CagA activates human phosphatase (SHP2) after binding to it, which subsequently serves as an oncoprotein that promotes cell growth. Hypergastrinemia, related to H. pylori colonization, has been suggested as a potential mechanism for tumorigenesis due to its trophic impact on the intestinal mucosa. Based on multiple investigations on this hypothesis, elevated circulating gastrin levels have been reported in H. pylori patients with colorectal cancer (43).
Gastrointestinal cancers, including gallbladder, anal, pancreatic, esophageal, colon, small intestine, gastric, colorectal, and liver, are some of the deadliest cancers in humans (6).
Regarding the relationship between H. pylori infection and EAC, H. pylori were significantly associated with reduced risks of EAC (6, 34). But the association between H. pylori and esophageal squamous cell carcinoma was not statistically significant (34). According to two meta-analyses, H. pylori infection was associated with a significantly reduced risk of EAC (6, 34). Some of the more reliable present hypotheses in this area are described below. First, H. pylori infection with gastric atrophy and destruction of parietal cells decreases the risk of reflux esophagitis and Barrett's esophagus. Second, H. pylori infection triggers the esophageal adenocarcinoma cell apoptosis, which travels from Barrett's esophagus through Fas- and caspase-mediated pathways.
According to Gao et al. (17), esophageal squamous cell carcinoma had no significant correlation with H. pylori infection in the general population. However, a population in the Middle East showed a significant correlation. Differences in risk factors between different regions may show diverse correlations, which can be attributed to different food cultures in different regions.
Pancreatic cancer (PC) is the fourth main cause of cancer mortality among men and women in the USA (44). Some reported risk factors for PC include old age, smoking, African-American race, type II diabetes, obesity, chronic pancreatitis, inherited syndromes, genetic mutations, and consumption of processed or smoked meat (45). Several meta-analyses showed conflicting results regarding the association between H. pylori and pancreatic cancer (4, 37). Contrary to the above meta-analyses, newer ones did not confirm this association (16).
There are limitations to the current overview. There was a difference in the methodology of the studies. First, studies had various eligibility criteria to select the participants. Second, the observational design of the studies included in the meta-analysis could not determine the causality. Third, the risk of bias was observed in several meta-analyses because the studies’ qualities were assessed according to the Newcastle-Ottawa Scale (NOS) version, and this scale is largely subjective. Fourth, conference abstracts and other gray literature were not included in some systematic reviews and meta-analyses, which could have increased the likelihood of publication bias. Fifth, there were differences in the control types (population-based, hospital-based, or other cancer controls). Sixth, different studies used different types of tests (serologic and histology detection methods, polymerase chain reaction (PCR) methods) to detect Hp infection.
Seventh, the meta-analyses included in the present overview had moderate to high heterogeneity. Although heterogeneity decreased after subgroup analysis in some meta-analyzes, it may have reduced the number of studies and limited the reliability of findings and the strength of the meta-analyzes. Eighth, some of the studies included in meta-analyses were not adjusted for potential confounding factors such as age, sex, country of birth, smoking status, educational level, physical activity, mean lifetime, body mass index, and diabetes in their research design or data analysis. Ninth, some studies have suggested the possibility of spontaneous disappearance of H. pylori infection with the progress of gastric atrophy or metaplasia, leading to false negatives and potentially influencing the outcomes. Multiple diagnostic techniques can help reduce the false negative results of H. pylori infection diagnosis (17).
4.1. Conclusions
H. pylori infection had a significant association with pregnancy complications such as gestational diabetes mellitus, preeclampsia, hyperemesis gravidarum, fetal growth restriction, birth defect, and iron-deficiency anemia during pregnancy, as well as some types of cancers such as colorectal, colorectal adenoma and lung malignancies. In esophagus cancer, H. pylori infection was significantly reduced in adenocarcinoma but not in esophageal squamous cell carcinoma. There are conflicting findings about the association between H. pylori infection and the risk of colorectal cancer and pancreatic cancer, and spontaneous abortion.