1. Background
Carpal tunnel syndrome is a common cause of upper extremity disability (1). It is a peripheral entrapment neuropathy because of the involvement of the median nerve in the wrist region.
The symptoms include pain, tingling, paresthesia, and discomfort, and as the disease progresses, hand movement will become impaired (2), paresthesia becomes more severe while driving, and symptoms are relieved by shaking the hand (3).
With a prevalence of 4% in the population, carpal tunnel syndrome is one of the most common entrapment neuropathy (4). The causes of CTS include medical conditions (thyroid disease, kidney failure, acromegaly, etc.), inflammatory disease (lupus, rheumatoid arthritis, etc.), tumors and pseudo-tumors (ganglions, etc.), anatomical anomalies, and in most cases, it is idiopathic (5).
The disease occurs with increased pressure in the carpal tunnel, especially at the level of the carpal ligament, which results in pressure on the median nerve, decreased epineural blood supply to the nerve, and progression of nerve damage due to local ischemia (6).
Neurophysiologic studies (such as nerve conduction studies) are highly sensitive diagnostic tests that investigate median nerve involvement (7). The degree of a patient's symptoms and level of disability can be assessed using reliable patient-centered tools like the Boston Carpal Tunnel Syndrome Questionnaire (BCTQ). The correlation between clinical findings and nerve conduction study findings allows for assessing the patient's level of disability (8).
Both surgical and non-surgical alternatives are available for treating carpal tunnel syndrome. Physical agent modalities are one of the various non-surgical treatments for carpal tunnel syndrome (7, 9).
Diathermy is one of these modes. Physical therapy uses diathermy, a deep heat current source that generates a high current in the body. Several persistent musculoskeletal issues are treated with diathermy (10).
Recently, a new system has been created to transfer resistive and capacitive energy called TECAR therapy®. This type of diathermy increases the tissue's intrinsic metabolism through inter-tissue energy transfer without releasing energy to the outside. The TECAR device works based on the physical principles of the condenser and consists of two pads separated from the device, placed on the surface, and made of insulating material. One of the two electrodes, which is active, is in charge of transferring energy to the tissue. These two electrodes are connected to the energy-generating device, and a flow between them is established (10, 11). Effects of resistance-capacitance diathermy, also known as TECAR therapy, have increased microcirculation flow, vasodilation (increased oxygenation), and tissue heat (11, 12).
2. Objectives
Studies evaluating the efficacy of TECAR therapy in the treatment of peripheral neuropathies, particularly entrapments of peripheral nerves, are rarely available, although numerous studies have been conducted to date to investigate the effectiveness of TECAR therapy in various diseases. One of the pioneering studies on using TECAR therapy as an innovative physical therapy modality to treat median nerve entrapment at the wrist (carpal tunnel syndrome) was conducted in this study.
3. Methods
3.1. Trial Design
The present study is a single-blind randomized clinical trial performed in neuromuscular clinics affiliated with the Department of Physical Medicine and Rehabilitation of Isfahan University of Medical Sciences in 2022. Patients presenting with complaints in their hands, such as pain, paresthesia, numbness, or weakness, were approached with a detailed history and a complete physical examination, including special tests for CTS such as Compression, Tinel, Phalen, and Reverse Phalen tests. The patients were then chosen to receive an EMG-NCV neurophysiological evaluation to confirm the diagnosis of CTS and rule out any other potential diagnoses. Mild and moderate cases of CTS that met the criteria of the American Association of Electrodiagnostic Medicine's guidelines (13) were included in the study. A peak latency greater than 3.5 milliseconds and NCV across wrist < 40 m/s was considered mild, and an average motor onset latency greater than 4 milliseconds was considered moderate. The EMG-NCV was taken by the physical medicine specialist of Isfahan University of Medical Sciences with a Natus device (UltraPro S100, Natus Neurology Incorporated 3150 pleaseant view road Middleton, WI USA).
Other inclusion criteria were age over 18 and under 60 years old and unilateral or bilateral idiopathic carpal tunnel syndrome lasting more than a month. The following criteria for exclusion were used: the presence of both systemic and local diseases (such as diabetes, RA, wrist arthritis, hypothyroidism), current or past cancer, use of a splint within the previous three months, pregnancy, burns to the hand or forearm, use of a pacemaker, prosthesis, or IUD, peripheral vascular diseases, fractures in the wrist and hand region, and corticosteroid injection in the carpal tunnel within the prior three months (14).
3.2. Interventions
Both groups (1 and 2) were treated for four weeks with a wrist splint of thermoplastic type in 0 to 5 degrees of the extension during the nights and during activity in the daytime plus 300 mg of vitamin B1(Vitamin B1-SHAFA 300 MG) tablets. In the intervention group (group 1), TECAR therapy was also performed with the WINBACK 3 device made in France, with a frequency of 500 Hz and intensity of 30 to 50% and medium Capacitive Energy Transfer electrode (60 mm), from the proximal carpal tunnel to the palm Median nerve path, while the active capacitive electrode was moved by an operator in a circular pattern and the passive electrode fixed on the dorsum of the hand, in two sessions per week for four weeks (Figure 1).
3.3. Outcomes
At baseline, the VAS and Boston questionnaires were completed for both groups. After 2 months of the treatment, the questionnaires were filled out again. VAS scale (15) is a 10 cm graduated line whose numbers are graded from 0 (absence of pain) to 10 (the most severe pain possible). In this scale, the patient circles a number to determine its score. This scale has been widely and comprehensively used in research related to pain, and its validity and reliability have been confirmed (16).
Levine designed for the first time in 1993 the Boston Questionnaire to determine the severity and quality of carpal tunnel syndrome, which includes 2 sections on symptom severity and functional status in people with carpal tunnel syndrome (17). Each question has a maximum of five and a minimum of one point, and the average score is obtained by dividing the total points by the number of questions. Rezazadeh et al. (18) corroborated the validity and reliability of the Boston questionnaires among the Iranian population.
Clinical tests and NCV parameters (Median antidromic sensory peak latency, sensory nerve conduction velocity (NCV) across the wrist, Median motor distal onset latency) were also examined and compared before and after treatment.
3.4. Sample Size
According to the formula for comparing means in clinical studies, considering type 1 error of .05, study power of 80%, d = 1.52, and S = 1.7 (for VAS variable in previous studies (19)) for the interpretation of the result for the superiority of TECAR therapy combined to conventional treatment over conventional treatment, the final sample size was determined 25 hands in each group.
3.5. Randomization
The participants were enrolled using a consecutive sampling method. Random assignment was done via an online random sequence generator.
3.6. Blinding
In this single-blind research, the clinician and the patients were aware of the intervention groups. However, the outcome assessor and the statistical consultant were kept blind to each patient's intervention group.
3.7. Statistical Methods
The collected data were analyzed using SPSS version 26. Appropriate parametric and nonparametric tests, including the paired-sample t-test (Boston-SSS and FSS variables and electrodiagnostic parameters in within-group comparison), independent t-test (Boston-SSS and FSS variables and electrodiagnostic parameters in between groups comparison), chi-square test (Demographic table variables), McNemar and Fisher exact test (Compression, Tinel, Phalen and Reverse Phalen variables within and between groups), Wilcoxon, and Mann-Whitney U test (VAS variable within and between groups), were used for statistical analyses. P-value < 0.05 was considered as the significance level.
4. Results
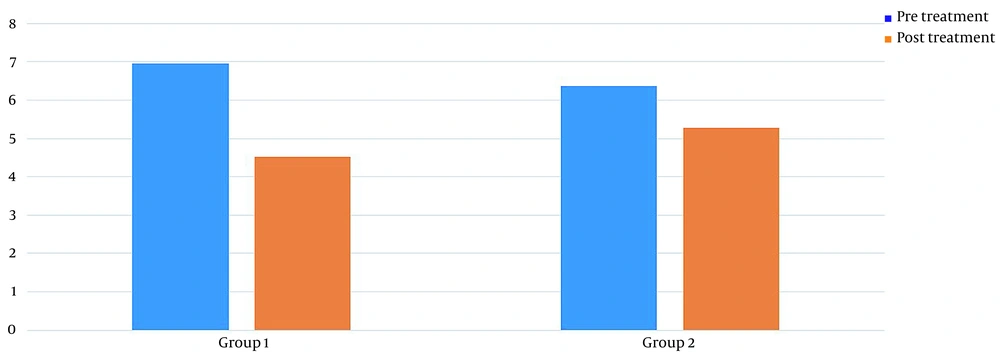
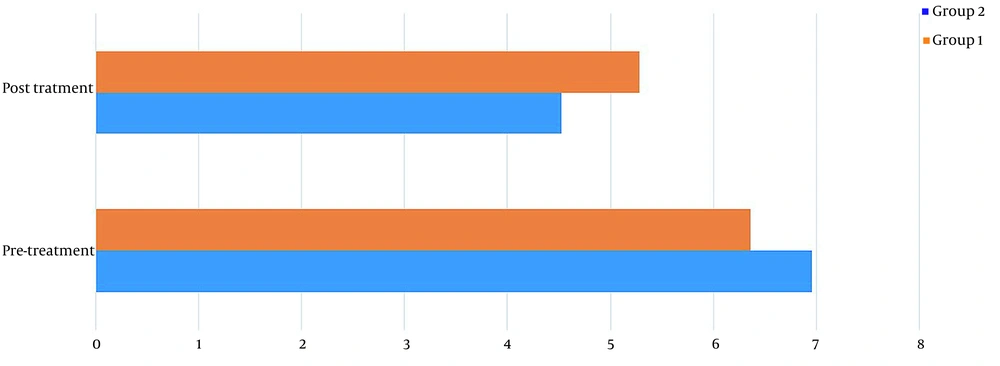
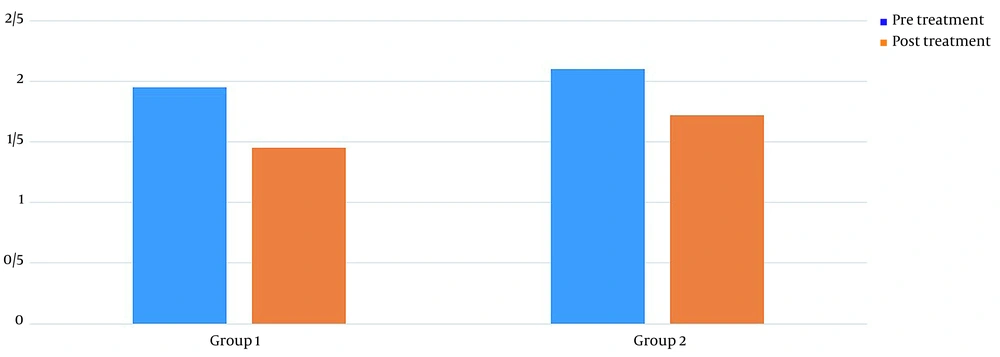
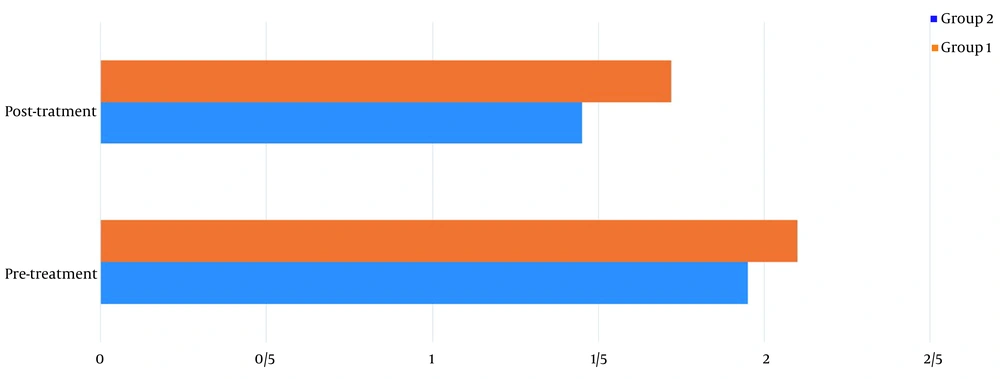
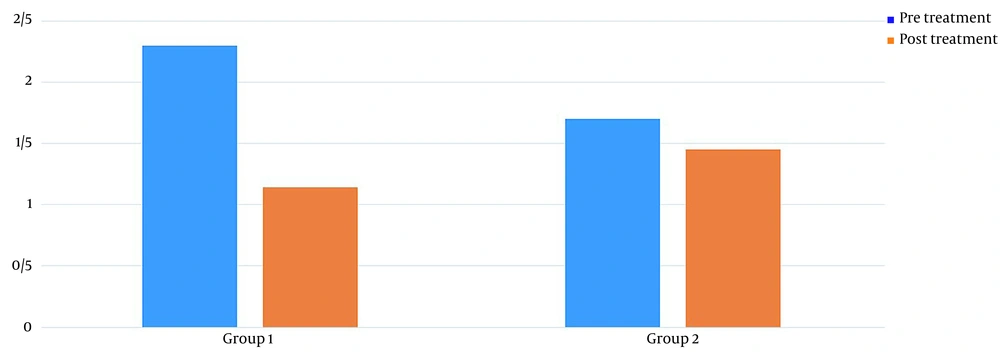
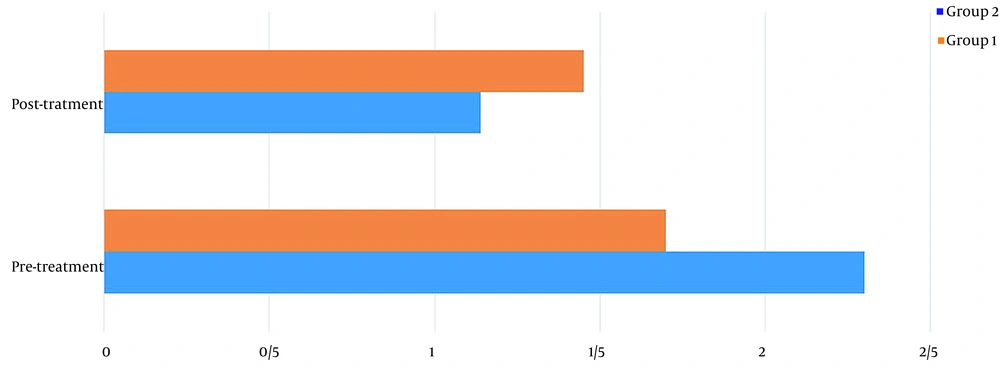
Table 1 shows the demographics of the study participants. Before treatment, there was no significant difference in pain levels between the two groups. Two months after treatment, in both groups, patient pain levels were significantly reduced (P < 0.001) (Figure 2). If the reduction of 25% of pain level (20) is considered significant, group 2 (wrist splint) didn't reach MCID (a minimal clinically important difference). There was a significant difference in pain reduction between the two groups, with a higher recovery rate in the TECAR treatment group (P = 0.008) (Figure 3). After two months of treatment within groups, the difference became significant in all special clinical tests (Tinel (0.002), Compression (0.004), Phalen (0.031), and reverse Phalen (0.031)) in the TECAR group and Compression (0.031) and Reverse Phalen (0.016) tests in the wrist splint group. But between the groups, clinical tests did not show a significant difference. Before the study, there was no significant difference in BCTQ (FSS and SSS) variables between groups (P = 0.740). Two months after the treatment, a significant difference was seen within (P = 0.018, P = 0.01) and between groups (P = 0.003, P = 0.014) (Table 2 and Figures 4 - 7). In our study, the wrist splint group didn't reach for both SSS and FSS to cut off level, but in the TECAR group, SSS was significant in MCID level (21), but FSS not significant.
| Group 1 (TECAR + Splint) (n = 14, 25 Wrist) | Group 2 (Splint) (n = 15, 25 Wrist) | P-Value | |
|---|---|---|---|
| Age | 47.08 ± 8.81 | 44.92 ± 10.20 | 0.678 |
| Gender(f/m) | 19/6 | 18/7 | 0.530 |
| Affected wrist | 0.321 | ||
| Right | 2 | 4 | |
| Left | 1 | 1 | |
| Both | 11 | 10 | |
| Neurophysiological class | 0.620 | ||
| Mild | 14 | 12 | |
| Moderate | 11 | 13 |
| Group 1 (TECAR + Splint) | Group 2 (Splint) | 95% Confidence Interval of the Difference | F | P-Value (Mann-Whitney) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| VAS | ||||||
| Pre | 6.96 ± 1.01 | 6.36 ± 1.15 | -0.01820 | 1.21820 | 0.0565 | 0.057 |
| Post | 4.52 ± .96 | 5.28 ± .97 | -1.31235 | -0.20765 | 0.019 | 0.008 |
| P-value (Wilcoxon) | < 0.001 | < 0.001 | ||||
| P-value (Independent t-test) | ||||||
| Levine-Boston/SSS | ||||||
| Pre | 2.30 ± .0.44 | 1.70 ± 0.48 | 3.628 | 9.492 | 0.111 | 0.740 |
| Post | 1.14 ± 0.15 | 1.45 ± 0.46 | -5.571 | -1.228 | 18.818 | 0.003 |
| P-value (Paired-samples t-test) | 0.012 | 0.011 | ||||
| Levine-Boston/FSS | ||||||
| Pre | 1.95 ± 0.48 | 2.10 ± 0.44 | -03.322 | 0.921 | 0.658 | 0.261 |
| Post | 1.45 ± 0.36 | 1.72 ± 0.38 | -3.871 | -0.448 | < 0.001 | 0.014 |
| P-value (Paired-samples t-test) | 0.018 | 0.01 | ||||
| P-value (Fisher's Exact Test) | ||||||
| Tinel test (positive/negative) | ||||||
| Pre | 17/8 | 16/9 | 1.000 | |||
| Post | 7/18 | 12/13 | 0.378 | |||
| P-value (McNemar test) | 0.002 | 0.063 | ||||
| Compression test (positive/negative) | ||||||
| Pre | 16/9 | 16/9 | 0.010 | |||
| Post | 8/17 | 10/15 | ||||
| P-value (McNemar test) | 0.004 | 0.031 | 0.194 | |||
| Phalen test (positive/negative) | ||||||
| Pre | 13/12 | 15/10 | 0.015 | |||
| Post | 9/16 | 12/13 | ||||
| P-value (McNemar test) | 0.031 | 0.063 | 0.393 | |||
| Reverse Phalen test (positive/negative) | ||||||
| Pre | 15/10 | 17/8 | 0.081 | |||
| Post | 9/16 | 10/15 | 0.667 | |||
| P-value (McNemar test) | 0.031 | 0.016 | ||||
Before the intervention, there was no discernible difference between the two groups according to neurophysiological tests (median sensory peak latency, median motor distal onset latency, and sensory NCV across the wrist). Also, there was no significant clinical difference between and within the groups two months after the treatment, according to the tests (Table 3).
| Group 1 (TECAR + Splint) | Group 2 (Splint) | 95% Confidence Interval of the Difference | F | P-Value (Paired Sample t-Test) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Median sensory PL (millisecond) | ||||||
| Pre | 4.195 ± 0.322 | 4.170 ± 0.450 | -0.197 | 0.248 | 2.179 | 0.818 |
| Post | 4.089 ± 0.284 | 4.130 ± 0.449 | -0.256 | -0173 | 4.843 | 0.700 |
| P-value (Independent t-test) | < 0.001 | < 0.001 | ||||
| Median motor OL (millisecond) | ||||||
| Pre | 4.209 ± 0.552 | 4.194 ± 0.448 | -0.270 | 0.301 | 1.627 | 0.913 |
| Post | 4.147 ± 0.510 | 4.098 ± 0.400 | -0.212 | 0.309 | 2.087 | 0.709 |
| P-value (Independent t-test) | 0.113 | 0.136 | ||||
| NCV across wrist (meter/second) | ||||||
| Pre | 33.48 ± 5.94 | 34.72 ± 5.20 | -4.414 | 1.942 | 0.221 | 0.438 |
| Post | 33.92 ± 5.99 | 35.12 ± 5.23 | -4.825 | 1.545 | 0.237 | 0.306 |
| P-value (Independent t-test) | 0.048 | < 0.001 | ||||
5. Discussion
In this study, we assessed the impact of TECAR therapy on electrodiagnostic parameters and clinical symptoms in patients with mild to moderate CTS.
In our study, VAS and Boston Questionnaire variables showed a good response, but NCV parameters did not show any specific changes from a clinical point of view. Perhaps the reason for the lack of clear changes in the NCS changes is that there is not enough time for changes to show. However, we can justify the VAS and Boston Questionnaire variables by examining the research and article review mechanism.
Few studies have been done on TECAR therapy, a novel deep heat physical modality, as a possible treatment for peripheral entrapment neuropathies or neuropathic pain.
The mechanism of action of TECAR has not been elucidated. The articles confirmed reduced muscle spasms, increased oxygenation, improved metabolic status, and reduced pain. By increasing radiofrequency energy to tissues, this treatment relaxes muscles and ligaments and improves nervous system repair (22). The main complaint of CTS patients is usually pain, which was measured by the VAS scale in this study. According to the Gate theory processes and mechanisms described above, reductions in VAS scales and patient pain levels are justifiable.
Lindblad et al. (23) investigated the efficacy of TECAR therapy for chemotherapy-induced neuropathic pain. Although TECAR therapy reduced patients' pain perception, the mean pain Numeric Rating Scale (NRS) did not reveal a discernible difference between groups. In the Lindblad study, using the interferential therapy with high-power TECAR didn't result in significant pain relief in the intervention group; however, from the gate control theory, we expected some more pain reduction; this may be because the disease is chronic, and resistant to treatment, and different from a focal entrapment neuropathy. M. Niajalili et al. (24) evaluated the effectiveness of TECAR therapy in diabetic patients with peripheral neuropathy. Both groups (Group 1=TECAR + Infrared and Group 2=Infrared + Sham TECAR) experienced an improvement in pain management, with the intervention group experiencing greater recovery. It suggests that the cause of the neuropathy may influence the TECAR therapy's effectiveness. In the field, however, more research is necessary.
Also, TECAR's action can be compared to other deep heat modalities. Ebenbichler et al. (25) studied the efficacy of therapeutic ultrasound in patients with mild to moderate CTS. They showed improved clinical symptoms, and NCS parameters were also observed at the 6-month follow-up. Also, Huisstede et al. (26) reported no short-term improvements in their systematic review of ultrasound effects, but long-term effects were seen. We also found that TECAR therapy was effective in improving clinical symptoms but no NCS parameters at 2 months, during which long-term follow-up was not performed.
Possibly one of the reasons for reducing patient symptoms and improving performance is the reduction of edema in the carpal tunnel region. One of the pathological causes of this disease is increased pressure in this area, so by increasing lymphatic outflow and reducing pressure on nerves, gradual improvements in patient symptoms can be observed (27).
Another deep heat modality used for CTS treatment is shortwave diathermy (SWD). In a study by Incebiyik et al. (28), SWD has shown pain relief, improved hand function, and CTS clinical testing, but electrodiagnostic parameters have not been studied. In our study, the pain decreased, and hand function improved in the treatment group, but we observed no important changes in clinical tests compared to the control group.
Frasca et al. (6) conducted a study in 2011 to evaluate the efficacy of microwave diathermy (MWD) in sham and treatment groups. They found a reduction in VAS for pain and an improvement in the Boston Carpal Tunnel Questionnaire but no electrodiagnostic changes. In our study, an electrodiagnostic improvement was observed in addition to the improvements observed in the previous study.
Among other interventions in managing CTS, corticosteroid injections into the carpal tunnel revealed primary evidence of efficacy in a systematic review by Piazzini et al. (29). Also, Visser et al. (30) showed that corticosteroid injection therapy is more effective in patients with mild CTS. In our study, sensory nerve conduction showed more changes than motor studies, but no significant differences were found between the two groups and clinically within the groups. This fact may indicate that patients with mild CTS respond better to treatment, but our study did not examine patients with mild and moderate CTS separately.
5.1. Study Limitations
Because of COVID-19, the study was conducted with a small sample size, and no follow-up was performed due to time constraints. However, this study points to the need for longer follow-ups.
5.2. Conclusions
Our results indicated that TECAR could effectively treat mild to moderate CTS patients. To investigate this, future interventional studies are required. It is better to conduct a study with a larger sample size and a longer follow-up to examine the changes in NCS from a clinical point of view.