1. Background
Cancer is one of the three leading causes of death worldwide (1). Sexual and reproductive health is an important component of life quality, which can affect patient satisfaction (2). Ovarian cancer (OC) is one of the most lethal malignancies of the female reproductive system (3). Epithelial ovarian cancer is one of the leading causes of death from gynecological cancers in the Western world (4), and only about 40% of women with ovarian cancer survive 5 years after diagnosis (5). In Iran, ovarian cancer is the eighth most common cancer, with an age-standard incidence of 3.9/100,000 (6). Although the five-year survival rate has increased in the last decade, the low survival rate causes recurrence (7). Ovarian cancer often does not have a specific symptom in its early stages, so it is spread at diagnosis (8).
Methods based on tree models, unlike classical methods, require fewer assumptions and include a wider range of data, so in the last two decades, these methods have become more popular than classical models. In particular, these models fit well with high data volumes, and the classical models have no problems with missing data. They also do not require the strict preconditions of common regression models, such as the normal distribution and homogeneity of variances (9). A decision tree is one of the nonparametric methods for classifying data that, according to the nature of the dependent variable, is divided into two categories, a classification tree for classification variables and a regression tree for continuous variables (10). Classification and regression tree with the growth of the largest tree creates a sequence of trees and prunes it so that only the root node remains. Then it uses cross-validation to estimate the incorrect classification cost of each subtree and selects the tree with the lowest estimated cost (11). The classification and regression tree (CART) considers the values of the predictors sequentially, meaning the variables are arranged according to their importance (12).
In a study conducted in China in 2018 to determine the effect of age on the survival of head and neck cancers, the classification and regression tree showed that the relative importance of age was 3.21% for oral cancer, 8.32% for oropharyngeal cancer, 2.56% for hypopharyngeal cancer and 16.51% for laryngeal cancer (13). In another study using the decision tree to determine lung cancer predictors in 2010, the best predictor by using CART was exposure to known lung carcinogens, the second predictor was 8.6 years or higher latency, and the third predictor was the smoking history of fewer than 11.25 packages in a year (14). In another study conducted in China in 2012 on non-small-cell lung cancer patients treated with gefitinib after chemotherapy, a classification and regression tree formed four subgroups, and the median PFS in the four subgroups ranged from 12 to 42 months (15).
2. Objectives
Many studies have investigated the factors affecting the survival or recurrence of ovarian cancer. However, so far, no study using the Classification and Regression Tree (CART) has investigated the recurrence rate and factors affecting the recurrence of epithelial ovarian cancer. We investigate the factors affecting the first recurrence of epithelial ovarian cancer over an 11-year period using the CART tree.
3. Methods
3.1. Participants
We considered a set of variables that were predictors of the first recurrence of epithelial ovarian cancer. This retrospective study reviewed medical and chemotherapy records of patients with ovarian cancer who had been referred to the oncology and radiotherapy department of Imam Hossein Hospital in Tehran between 2007 and 2018. Therefore, we did not need to determine the sample size, and the sampling was a census of the patients referred to Imam Hossein Hospital in Tehran, which we can consider as a sample of the entire population of epithelial ovarian cancer. The data were extracted from the patient's files in the hospital's archives.
All patients underwent the first stage of treatment, including the first stage of chemotherapy, after the initial cancer diagnosis. Recurrence of tumor masses in any part of the body had been detected by imaging, with or without biopsy, but it had usually been detected by the tumor marker CA125, and chemotherapy had restarted after recurrence. So the recurrence time for patients who had experienced recurrence was between the end of the first chemotherapy period and the restart of later chemotherapy. For patients who had been censored, the time of censoring was recurrence time, and for patients who had not experienced recurrence to the end of follow-up, time to the end of follow-up was considered time to censor. The first recurrence by the end of 2018 was considered a failure.
Potential prognostic variables accessed in this study included clinical variables such as age, body mass index, presence of ascites at diagnosis, tumor-related variables including tumor stage, tumor grade, metastatic tumor, tumor histology, tumor size, CA125 tumor marker at diagnosis, and hematologic variables such as white blood cells, platelets, hemoglobin, type of chemotherapy (adjuvant or neoadjuvant) and chemotherapy courses.
3.2. Statistical Analysis
To determine the effect of the variables under study on the first recurrence of epithelial ovarian cancer, we defined time to first recurrence as a dependent variable. To calculate the median time to the first recurrence, the time to the first recurrence was calculated for all patients, and then the median was calculated from these times. Because the time until the first recurrence is a continuous variable, the regression tree was used to determine the importance of the variables in the time until the first recurrence. In the regression and classification tree, the dependent variable is placed in the tree leaves, so the time to the first recurrence is reported in the leaves, and we can calculate the shortest time to the first recurrence and the path from the root to these leaves. This path includes patients with specific clinical characteristics.
In the classification tree, due to the fact that the dependent variable must be a classification variable, it was considered to have the first recurrence and censoring as a classification variable. In leaves is reported probability the first recurrence and based on it having recurrence or censoring have belonged them. In this work, instead of having or not having recurrence was reported the probability of recurrence in the leaves. The leaves can contain different values of the probability of recurrence, based on which the highest probability of recurrence and its path from the root to the leaf can be reported that include a certain class of patients. Statistical analyses were performed using R (Ver 3.4.3). P ≤ 0.05 was considered as statistical significance.
4. Results
4.1. Descriptive Results
In this retrospective study, 141 eligible patients were included in our follow-up from 2007 to 2018; 58 (41%) patients had a first recurrence in our follow-up, and the rest either did not experience the first recurrence or were censored during this period (59%). So the median time to the first recurrence in these patients was 17 (0.5 - 0.57) months, and the median age of the patients was 52 (23 - 82) years. Disease-free survival was 0.82 for one year, 0.55 for two years, 0.44 for three years, 0.42 for four years, 0.39 for five years, and 0.36 for ten years. Table 1 and Table 2 show the properties of the selected continuous and discrete variables, respectively.
| Continuous Variables | Range | Mean | Median |
|---|---|---|---|
| Age, y | 23 - 82 | 52.7 | 52 |
| BMI, kg/m2 | 12.66 - 39.45 | 27.15 | 27 |
| Baseline CA125 | 8 - 4900 | 619 | 200 |
| Minimum platelet count | 76000 - 410000 | 152950 | 135000 |
| Maximum platelet count | 178000 - 819000 | 381000 | 353000 |
| Mean platelet count | 126000 - 516000 | 251190 | 232750 |
| Mean white blood cells | 3065 - 10627 | 5192 | 4965 |
| Mean hemoglobin | 8.36 - 13.23 | 10.87 | 11 |
| Minimum hemoglobin | 7.3 - 12.8 | 9.66 | 9.6 |
| Size of the primary tumor, cm | 1 - 26 | 10.52 | 9.75 |
| Categorical Variables | No. (%) |
|---|---|
| Metastatic tumor | |
| Yes | 52 (36.9) |
| No | 80 (56.7) |
| Tumor grade at diagnosis | |
| Grade I | 24 (17) |
| Grade II | 32 (22.7) |
| Grade III | 37 (26.2) |
| FIGO stage at diagnosis | |
| Stage I | 34 (24.1) |
| Stage II | 16 (11.3) |
| Stage III | 44 (31.2) |
| Stage IV | 14 (9.9) |
| Baseline ascites | |
| Presence of ascites | 58 (41.1) |
| No presence of ascites | 78 (55.3) |
| Chemotherapy course | |
| Three weeks | 64 (45.4) |
| One week | 37 (26.2) |
| Adjuvant chemotherapy | |
| Adjuvant | 64 (13.5) |
| Neoadjuvant | 19 (45.4) |
| Tumor histology | |
| Papillary serous | 85 (60.3) |
| Others (endometrioid, clear cell, mucinous) | 30 (21.2) |
4.2. Survival Analysis Using the CART Method
4.2.1. Regression Tree
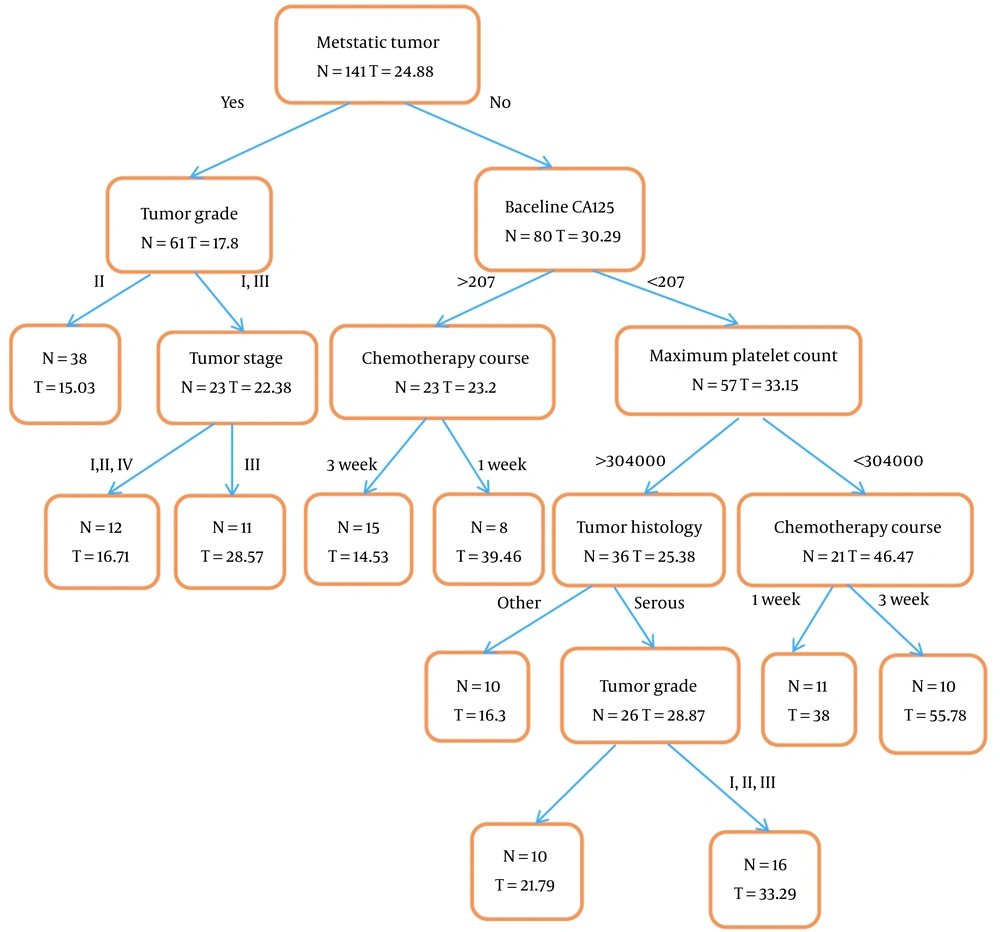
Terminal nodes in the regression tree were time to the first recurrence of epithelial ovarian cancer. The root of the regression tree was based on metastatic tumors so that the shortest time to first recurrence in patients with metastasis was among patients with grade 2 tumor (15.03 ± 11 months), also in patients without metastasis, the shortest time to the first recurrence was among patients who had a CA125 tumor marker at diagnosis above 207 and used 3-week chemotherapy courses (14.53 ± 6.4 months). The number of individuals and the mean time to the first recurrence is specified in all nodes of the regression tree, including the final nodes, as shown in Figure 1.
4.2.2. Classification Tree
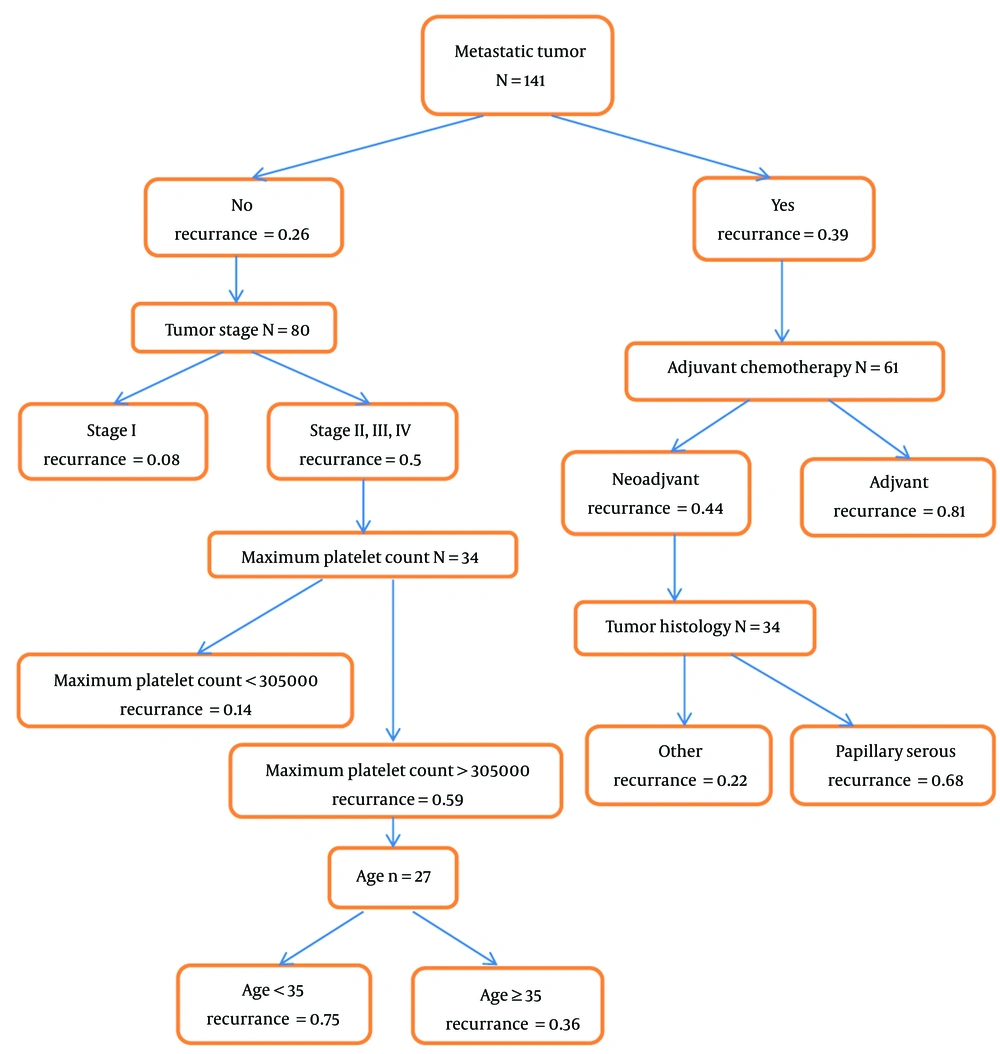
Having or not having the first recurrence reported in terminal nodes of the classification tree during our study, our response was two-state. The root of the classification tree is based on the metastatic tumor. In this tree, the number of individuals and the probability of the first recurrence in each node has been determined, especially in the final nodes was reported the probability of the first recurrence, so the probability of the first recurrence in each specific class of patients through the classification tree was reported (in this tree don't report time to recurrence). The results are shown in Figure 2. Descriptive statistics were reported in the nodes and leaves of the tree. So the risk of the first recurrence is highest among patients without metastasis who received adjuvant chemotherapy (0.81). Also, the highest probability of the first recurrence in patients without metastases was among patients with stages 2,3, and 4 with a maximum platelet count above 305,000 and less than 35 years old (0.75).
The variables' importance in the first recurrence of epithelial ovarian cancer using the classification and regression tree model is shown in Table 3. According to the regression tree results, chemotherapy courses with 17% significance and metastatic tumor with 14% significance were in the first and second category, respectively, and according to the results of the classification tree, tumor stage with 21% significance and metastatic tumor with 15% importance are in the first and second category.
| Regression Tree | Classification Tree | ||
|---|---|---|---|
| Variables | Importance, % | Variables | Importance, % |
| Chemotherapy course | 17 | FIGO stage at diagnosis | 21 |
| Metastatic tumor | 14 | Metastatic tumor | 15 |
| Tumor grade at diagnosis | 10 | Tumor histology | 14 |
| Baseline CA125 | 9 | Tumor grade at diagnosis | 12 |
| Maximum platelet count | 9 | Adjuvant chemotherapy | 10 |
| FIGO stage at diagnosis | 9 | Age | 7 |
| Age | 8 | Maximum platelet count | 6 |
| Mean platelet count | 6 | Mean platelet count | 5 |
| Adjuvant chemotherapy | 6 | Chemotherapy course | 5 |
| Tumor histology | 6 | Baseline ascites | 3 |
| Minimum hemoglobin | 3 | Minimum hemoglobin | 1 |
| Mean hemoglobin | 2 | ||
| Baseline ascites | 1 | ||
5. Discussion
In our study using the regression tree, the shortest time to the first recurrence in metastatic patients was among patients with grade 2 tumor (15.03 months), but in patients without metastasis, the shortest time to the first recurrence was among patients that had the tumor marker CA125 above 207 at diagnosis and used three-week chemotherapy courses (14.53 months). Also, using the classification tree, the risk of first recurrence in metastatic patients that received adjuvant chemotherapy is the highest possible. But patients without metastases at stages 2, 3, and 4, with a maximum platelet count above 305,000, and those under 35 years old had a higher risk of recurrence. In both the regression and classification trees, the metastatic tumor was identified as an important risk factor for the first recurrence of epithelial ovarian cancer.
In our study, the median time to the first recurrence of epithelial ovarian cancer among patients referred to Imam Hossein Hospital in Tehran was 17 (0.5 - 127) months, and the median age of patients in our study was 52 (23 - 82) years old. In the study of Komura et al. in 2019, this median age in Japan was less than 59 years (16). In our study, tumor stage was the most important risk factor for epithelial ovarian cancer identified by the classification tree. In the Clarke et al. study in 2019 that examined long-term survival predictors of patients with grade III and IV serous ovarian cancer, the lower stage of the disease was significantly associated with long-term survival (17), which is consistent with our study. In our study in people without metastases, a tumor marker above 207 shortens the time to recurrence; a study in 2018 showed that higher levels of the CA125 marker tumor increase the probability of abdominal recurrence in high-grade serous ovarian cancer patients (18).
Various studies have investigated the factors affecting the diseases using classification and regression trees, but this method has not been used in ovarian cancer, so we used in this section other studies that apply classification and regression trees to other diseases, especially cancers. Based on a study by Saki Malehi et al. that used a decision tree to evaluate prognostic variables in the survival rate classification of patients with colorectal cancer. The decision tree model showed that disease stage at diagnosis, patient age at the time of diagnosis, tumor morphology, and disease severity are important prognostic factors in the survival of patients with colorectal cancer (19).
Navarro Silvera et al.'s study in 2014 that investigated diet and lifestyle as risk factors in patients with gastric and esophageal cancer showed that the frequency of symptoms of gastroesophageal reflux disease was reported to be the most important risk factor. For esophageal cell cancer, smoking was the most important risk factor (20). In 2019 Greene et al. used classification and regression trees to predict cervical cancer screening; this model identified subgroups with the probability of receiving screening and several new variables that may underlie the use of SMW in cervical cancer screening (21).
5.1. Limitations
One disadvantage of classification and regression trees is that the CART tree divisions are binary, and when the number of variable levels is greater than two, the results can be confusing.
5.2. Conclusions
When the number of predictor variables is high, due to the interaction effects of the variables, regression methods are not very suitable. Classification and regression tree models without the need for none specific assumptions can predict the recurrence probability of different subgroups. These models do not require special knowledge due to ease of interpretation and can be easily used by physicians and paramedics.