1. Background
Spinal anesthesia is the choice method of anesthesia for scheduled Cesarean delivery. Maternal hypotension is a common complication of spinal anesthesia, which can occur in up to 80% of Cesarean deliveries (1, 2). Hypotension can cause maternal and fetal/neonatal adverse effects (3). In most cases, maternal side effects include nausea, vomiting, pallor, and shivering. However, severe complications, such as altered consciousness and respiratory and cardiac collapse, can also occur (2, 4). Prolonged and severe maternal hypotension decreases uteroplacental blood flow, leading to fetal acidosis and decreased neonatal Apgar scores (2, 4, 5). Prevention and management of hypotension consist of positioning protocols (left lateral tilt of the uterus), lower limb compression (leg wrapping or thromboembolic stockings), pharmacological agents (vasopressors), and fluid loading (colloids and crystalloids) (4, 6, 7). Animal studies reported the effectiveness of crystalloid coload in cases of hypotension during Cesarean section, with clear benefits for both mothers and newborns (8). In the recent literature, vasopressor administration has become the main method for hypotension prevention. However, intravenous fluid therapy is critical to hypotension management (1, 2). Fluid therapy decreases hypotension incidence and reduces vasopressor requirement (3, 9). Therefore, combining the mentioned strategies seems to be the best approach for preventing and managing hypotension.
Fluid therapy can be manipulated through volume, rate, fluid type, and timing. Fluids can be administered before spinal anesthesia (preload) or coincidently with anesthesia induction. Both crystalloid and colloid solutions can be administered in fluid therapy (4). Colloid solutions have been shown to have a superior effect compared to crystalloids in preventing hypotension in several studies (10, 11). However, recent studies have reported contradictory results (12, 13). In addition, administration of large volumes of colloid preload has been reported to have no additional benefits. It may also have adverse effects such as anaphylactoid reactions and renal function impairment (12, 14, 15). Based on a systematic review of the administration of both preloaded and concomitant crystalline and colloidal fluids, fluid therapy can reduce the incidence of hypotension; however, a vasopressor should be used for complete reduction (16).
Administration of intravenous glucose solutions before delivery has been shown to elevate maternal and embryonic energy and reduce neonatal hypoglycemia (17). A recent study showed that adding 1% glucose to the crystalloid solution before spinal anesthesia decreased postanesthesia complications in parturients undergoing Cesarean delivery. Still, studies investigating the effect of glucose-containing crystalloid infusions on maternal and neonatal outcomes are limited, and further investigations are required (5).
2. Objectives
The present study aimed to evaluate the impact of glucose-containing crystalloid infusion before anesthesia induction (preload) on maternal hemodynamics, blood glucose levels, neonatal Apgar scores, and postanesthesia complications.
3. Methods
3.1. Study Design and Sample Size
This study was designed as a prospective, double-blind, randomized clinical trial on 60 parturients scheduled for elective Cesarean delivery at Imam Reza and Ghaem hospitals of Mashhad University of Medical Sciences (MUMS), Mashhad, Iran, between August 2021 and September 2022. According to previous data from Rijs et al. (1), the incidence of hypotension was 10% and 45% in the study groups. Considering an alpha of 0.05 and a power of 80% based on the incidence of hypotension, a sample size of 30 per group was calculated.
3.2. Study Population
The participants were recruited from nonlaboring parturients aged 18 to 40 years, with term (gestational age of 38 - 40 weeks) uncomplicated singleton pregnancy and a physical status of ASA (American Society of Anesthesiologists) Grade I or II, who were scheduled for elective lower segment Cesarean section under spinal anesthesia. Those with gestational or pregestational diabetes, hypertension, cardiorespiratory disorders, cerebral disorders, fetal anomaly, or significant hemorrhage were excluded. The study protocol was approved by the Institutional Ethics Committee, and all the participants provided written informed consent.
3.3. Randomization and Blinding
The patients were randomly allocated to two groups using a block randomization procedure. A statistician not otherwise involved in this study performed the random allocation using http://www.sealedenvelope.com. The randomization lists were numbered consecutively and individually placed in opaque and sealed envelopes. The allocation assignment was revealed by opening the envelope on the day of the restorative intervention to prevent selection bias. The participants and the investigators were blinded to the group assignment.
3.4. Study Procedure
Standard monitoring, including pulse oximetry, non-invasive blood pressure, and electrocardiogram, was used in the operating room. The participants were randomly divided into the groups of glucose-containing normal saline (GcNS) and normal saline (NS). In both groups, 5 - 7 mL/kg of intravenous bolus serum was infused 15 - 20 minutes before spinal anesthesia. The participants in the GcNS group received 1% glucose solution in normal saline serum (10 g of glucose in 1000 mL), and the participants in the NS group received normal saline without the glucose solution. Spinal anesthesia was performed in the subarachnoid space at L3 - L4 or L4 - L5 intervertebral space with a 24 or 25-gaged needle in a seated position. A 2.2-mL solution containing 10 - 15 mg of hyperbaric bupivacaine 0.5%, plus 25 µg of fentanyl, was intrathecally injected.
3.5. Outcome Measurements
Systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), blood glucose concentrations (before spinal anesthesia, after operation), and neonatal Apgar scores were recorded. Complications such as nausea and vomiting, shivering, pallor, loss of consciousness, and respiratory distress were also recorded. Hypotension was defined as SBP < 100 mmHg or a > 25% decrease from baseline and was treated with intravenous administration of 5 - 20 mg ephedrine or 50 - 200 μg phenylephrine. Bradycardia was defined as HR < 50 beats/min and was treated with 0.5 mg of atropine (5). Consumption of vasopressors and atropine was recorded as well.
3.6. Ethics
This trial received ethical approval from the Ethics Committee of the Mashhad University of Medical Sciences (protocol ID: IR.MUMS.MEDICAL.REC.1401.021) and is registered with www.IRCT.ir (registration ID: IRCT20220417054563N1).
3.7. Statistical Analysis
We used the Kolmogorov–Smirnov test to detect the normality of the distribution of quantitative data. The quantitative variables were described as mean ± standard deviation (SD) and were compared using the independent t-test and Mann-Whitney U test. The qualitative variables were expressed as percentages and frequency and were compared with the chi-square and Fisher's exact tests. Repeated measures analysis of variance (ANOVA) was used to confirm the effects on the hemodynamics. All the statistical analyses were performed in the Statistical Package for the Social Sciences (SPSS) v. 16 (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered significant.
4. Results
Sixty patients were recruited (30 in each group), with a mean age of 29.14 ± 6.01 and 29.76 ± 6.15 years in the GcNS and NS groups, respectively. The mean weight in the GcNS and NS groups was 79.03 ± 9.92 and 79.70 ± 12.82 kg, respectively. Both groups were similar regarding age, sex, the number of children, and gestational age (Table 1).
Abbreviations: GcNS, glucose-containing normal saline; NS, normal saline.
a Values are presented as No. (%).
b Independent t-test.
c Chi-square test.
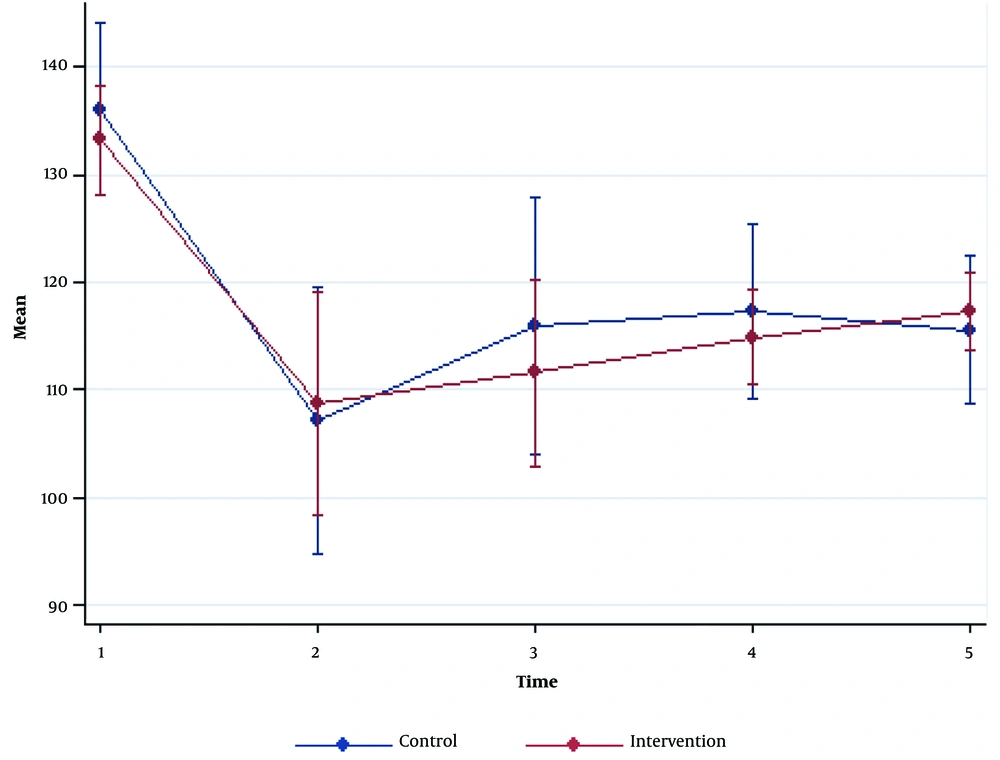
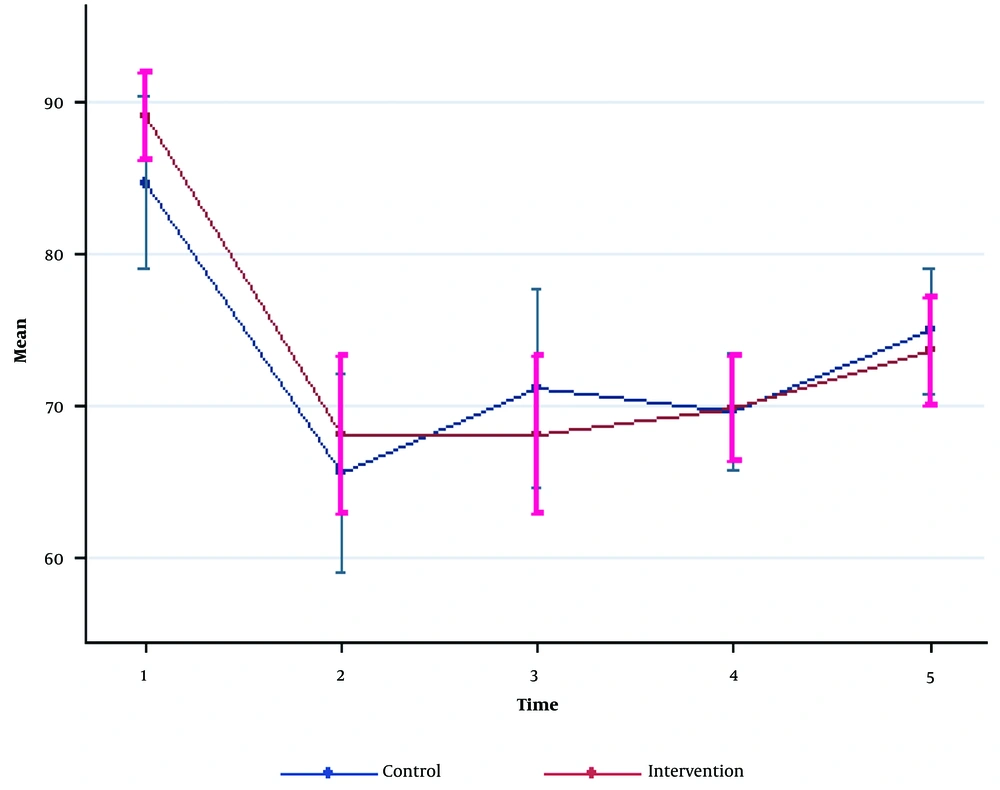
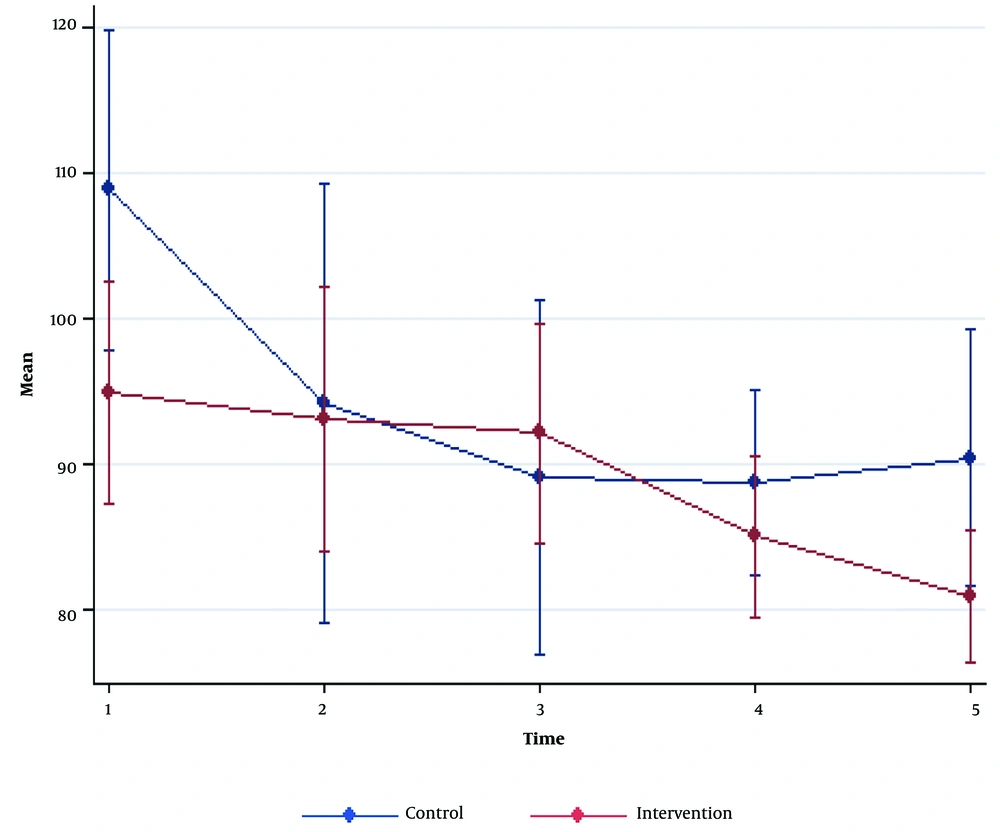
SBP and DBP reductions were seen in both groups after the induction of anesthesia. No significant differences were detected for SBP and DBP between the two groups (P > 0.05). HR was significantly higher at baseline and 30 minutes after the surgery in the NS group compared to the GcNS group (P = 0.005 and P = 0.01, respectively). However, the other time intervals had no significant differences (P > 0.05; Figures 1 - 3, Table 2).
| Variables | GcNS | NS | P-Value |
|---|---|---|---|
| Systolic blood pressure | |||
| Pre-anesthesia | 133.23 ± 10.04 | 136.1 ± 16.05 | 0.41 |
| 0 - 5 min post-anesthesia | 108.73 ± 20.67 | 107.1 ± 24.59 | 0.78 a |
| 5 - 10 min post-anesthesia | 111.53 ± 17.28 | 115.96 ± 23.65 | 0.41 |
| During operation | 114.83 ± 8.72 | 117.3 ± 16.24 | 0.46 |
| 0 - 30 min post-operation | 117.96 ± 7.20 | 115.56 ± 13.63 | 0.30 |
| P-Value | 0.78 b | ||
| Diastolic blood pressure | |||
| Pre-anesthesia | 88.96 ± 7.56 | 84.33 ± 15.25 | 0.16 a |
| 0 - 5 min post-anesthesia | 68.0 ± 13.96 | 65.56 ± 17.28 | 0.55 |
| 5 - 10 min post-anesthesia | 69.01 ± 13.87 | 71.06 ± 17.55 | 0.47 |
| During operation | 69.83 ± 9.18 | 69.56 ± 10.23 | 0.91 |
| 0 - 30 min post-operation | 73.56 ± 9.59 | 74.93 ± 11.02 | 0.61 |
| P-Value | 0.75 b | ||
| Heart rate | |||
| Pre-anesthesia | 94.80 ± 15.22 | 108.8 ± 21.87 | 0.005 a |
| 0 - 5 min post-anesthesia | 92.56 ± 18.04 | 94.13 ± 29.98 | 0.85 a |
| 5 - 10 min post-anesthesia | 92.06 ± 15.04 | 89.03 ± 24.33 | 0.56 a |
| During operation | 84.93 ± 11.00 | 88.7 ± 12.63 | 0.22 |
| 0 - 30 min post-operation | 80.86 ± 9.08 | 90.36 ± 17.48 | 0.01 |
| P-value | 0.74 b | ||
Abbreviations: GcNS, glucose-containing normal saline; NS, normal saline.
a Mann-Whitney U test.
b Repeated measures analysis of variance between groups after adjustment for the main variables at baseline.
Maternal blood glucose level was 77.33 ± 10.11 and 85.83 ± 14.69 in the GcNS and NS groups, respectively, and this difference was not significant (P = 0.19). The two groups had no significant difference in Apgar scores either (P = 0.12).
The incidence of complications of spinal anesthesia, including nausea and vomiting, pallor, and shivering, was higher in the NS group. However, only nausea and vomiting were statistically significant (P = 0.04). Loss of consciousness and respiratory distress were not reported among the participants in the two groups. Ephedrine and atropine consumption was higher in the NS group than in the GcNS group. Nevertheless, this difference was not significant (P > 0.05; Table 3).
Abbreviations: GcNS, glucose-containing normal saline; NS, normal saline.
a Values are presented as No. (%).
b Fisher's exact test.
5. Discussion
This was a prospective, double-blind, randomized clinical trial of 60 parturients undergoing elective Cesarean delivery under spinal anesthesia. We investigated the effect of 5 - 7 mL/kg of intravenous bolus infusion of 1% glucose solution in normal saline serum, as compared to normal saline, before anesthesia induction (preload) on maternal hemodynamics, blood glucose levels, neonatal Apgar scores, and post-anesthesia complications, including nausea and vomiting, shivering, pallor, loss of consciousness, and respiratory distress. Our results revealed a non-significant lower rate of hypotension in parturients receiving GcNS compared to NS. Moreover, nausea and vomiting 10 minutes after spinal anesthesia were significantly lower in the intervention group.
Spinal anesthesia often decreases blood pressure due to the sympathetic nervous blockade (18). Fluid therapy and vasopressor administration have been recommended to prevent hypotension following spinal anesthesia (4, 19-21). Crystalloid preloading has been the standard antihypotension strategy. However, recent studies have questioned its value and reported preloading with colloids to be more effective than crystalloids (3, 4, 22). In our study, the incidence of hypotension was lower in parturients receiving GcNS than NS (46.6% vs. 70.0%). However, this difference was not significant. In the literature, the incidence of hypotension ranges from 13% to 90% (5, 6, 12, 23-25). Most studies have compared the incidence of hypotension between preloading with colloids and crystalloids. Historically, colloid preloading has been more effective than crystalloid preloading in preventing hypotension (2-4, 10, 11, 26). Nevertheless, recent studies have reported controversial findings (13, 23, 27). In a randomized clinical trial of 80 parturients undergoing elective Cesarean section with spinal anesthesia, Atashkhoei et al. (5) reported that adding 1% glucose to a crystalloid solution (ringer lactate) improves hemodynamics. The incidence of hypotension was 75% in patients receiving a Ringer's solution with 1% glucose and 27.5% in patients receiving only a Ringer's solution in the mentioned study. Ringer's lactate infusion during Cesarean delivery is associated with an increased risk of metabolic acidosis compared to normal saline. Therefore, the present study used normal saline for intraoperative fluid therapy. In another randomized clinical trial with a prophylactic norepinephrine setting, hypotension occurred in 13.7% of the patients receiving colloid preload and in 16.3% of the patients receiving crystalloid coload during Cesarean section under combined spinal-epidural anesthesia (12). It is difficult to compare the hypotension incidence among studies due to the different definitions proposed in each study. Our study defined hypotension as SBP < 100 mmHg or a > 25% decrease from the baseline.
It has been reported that glucose administration during delivery provides maternal and embryonic energy and prevents ketone body increase (17). In our study, adding 1% glucose to the crystalloid solution did not significantly affect maternal blood sugar or neonatal Apgar scores. Similar to our results, previous studies reported no change in neonatal Apgar scores after the addition of glucose solutions to crystalloid infusions (5, 28). Atashkhoei et al. (5) and Fukuda et al. (17) also reported no significant difference in maternal blood sugar by adding glucose solutions to crystalloid infusions in parturients undergoing Cesarean delivery. A randomized clinical trial of 450 nulliparous women undergoing labor induction demonstrated that fluid therapy with Ringer's lactate, normal saline, or 1/3 - 2/3 fluids during labor was not associated with labor outcomes or glucose levels of the umbilical cord blood (29).
The present study demonstrated that post-operative complications, including nausea, vomiting, shivering, pallor, loss of consciousness, and respiratory distress, occurred less in patients receiving normal saline with 1% glucose solution than those receiving only normal saline. Still, this difference was not significant in most time intervals. Atashkhoei et al. (5) have also reported a lower incidence of complications such as sustained hypotension, agitation, nausea, pallor, and less consumption of ephedrine to treat complications in the group treated with glucose-added Ringer's lactate. Our results indicated a lower consumption of ephedrine and atropine in patients receiving normal saline with 1% glucose solution compared to those receiving only normal saline. However, this difference was not significant in our study. Further studies with larger samples are required to investigate the effect of adding glucose solutions to crystalloid preloading on hypotension and bradycardia incidence, complications, and the dosage of drugs administered to treat these complications. In our study, ephedrine and atropine were used to treat hypotension and bradycardia. The choice of ephedrine to treat hypotension in our study might be argued, as the literature has favored phenylephrine in treating hypotension after spinal anesthesia for Cesarean delivery (6). However, both vasopressors are shown to be safe and effective. Ephedrine was chosen in our study since it was the primarily used vasopressor in our institution (4).
The present study had some limitations that should be acknowledged. The small sample might have limited the results. We did not measure neonatal blood glucose levels or maternal cardiac output. Moreover, the participants were all uncomplicated singleton pregnancies scheduled for Cesarean delivery. Thus, our results cannot be generalized to complicated or unscheduled cesarean deliveries.
5.1. Conclusions
The present study did not justify the benefit of adding 1% glucose to normal saline solution preload on hypotension in parturients undergoing Cesarean delivery with spinal anesthesia. Although nausea and vomiting 10 minutes after spinal anesthesia were significantly lower in the intervention group, the lower incidence of hypotension and anesthesia complications in patients receiving glucose-added crystalloid were not significant in most intervals. Therefore, the efficacy of adding glucose solutions to fluid therapy remains to be determined and requires further investigation.