1. Background
Long-term administration and subsequent discontinuation of opioids, such as morphine, cause physical dependence and withdrawal syndrome, respectively. Withdrawal syndrome leads to neuronal excitotoxicity-related symptoms, such as agitation and seizure, induced by the primary excitatory amino acid neurotransmitter glutamate (i.e., one of the crucial molecular mechanisms involved in the pathophysiology of opioid withdrawal syndrome) (1). N-methyl D-aspartate (NMDA), as the main glutamate receptor, inhibitors have been shown to alleviate the physical dependence and the symptoms of opioid withdrawal (2), implicating that the maintenance of opioid dependence and withdrawal syndrome is established by glutamatergic transmission, especially via NMDA receptors (3). N-methyl D-aspartate-motivated calcium influx leads to the launching of neuronal injury induced by a biochemical cascade and worsens the cell’s conditions (4).
Adenosine (i.e., an organic and purinergic compound that exerts physiological and pharmacological roles via the stimulation of four known adenosine receptor subtypes [A1, A2A, A2B, and A3]), an endogenous inhibitory neuromodulator in the central nervous system (CNS), displays neuroprotective activity that is mainly based on the prevention of glutamate-induced neurotoxicity by the inhibition of its release through the activation of adenosine receptors particularly adenosine A1 receptor (A1R) (5, 6). Adenosine induces neuroprotection at least via two pathways: (1) The inhibition of the release of excitatory neurotransmitters, such as glutamate-mediated, by the presynaptic activation of A1Rs linked via G proteins to both Ca2+ and K+ ion channels (7-9); and (2) the activation of postsynaptic receptors that promote homeostatic potentiation of intracellular Ca2+ (10).
Unlike existing common neuroprotective medications, the body’s natural production of adenosine is not hampered by mechanisms in the brain that lead to drug resistance. Drug resistance can arise through structural or metabolic changes in the brain of unknown or genetic origin. Increased levels of transporter proteins in the blood-brain barrier and increased levels of proteins, such as P-glycoprotein (P-gp), in astrocytes and blood vessel cells might be nonspecific or adaptive mechanisms that lead to drug resistance. However, these mechanisms do not impact endogenous adenosine (11). Instead, this organic molecule still shows therapeutic effects against pharmacoresistant conditions, such as epilepsy, anxiety, and depression (12).
Japanese sake yeast (Saccharomyces cerevisiae or Japanese rice wine) has long been part of Japanese culture. Sake is a unique brewed alcoholic beverage made through a more complex fermentation process than most other alcoholic beverages (13, 14). Orally administered sake yeast contains adenosine analogs that can activate adenosine receptors and show potential neuroprotective effects. For instance, studies have shown that sake yeast supplements can improve sleep quality and promote non-rapid eye movement (NREM) sleep through the activation of adenosine A2A receptors in both clinical and animal experiments (15, 16). The most significant advantage of Japanese sake yeast supplement as an organic and natural agent, compared to the routine drugs available to treat withdrawal syndrome on the market (e.g., benzodiazepines), is that sake yeast shows no evidence of tolerance, dependence, addiction, or withdrawal symptoms. This suggests that it might have a lower risk of developing pharmacological resistance and more tolerability in patients.
Furthermore, orally administering sake yeast and its active ingredient, S-adenosyl methionine (SAM), might have advantages over injecting synthetic adenosine or adenosine A1R agonists. The ability to take sake yeast in oral powder or tablet form provides a non-invasive dosage option compared to injections. Not requiring intravenous delivery expands the practicality and convenience of sake yeast as a potential intervention. Finally, this study aimed at the oral administration of this novel natural agent to evaluate its neuroprotective effects on a mouse model of morphine withdrawal syndrome. The present study is the first to investigate the possible neurobehavioral protective effects of Japanese sake yeast against morphine withdrawal syndrome.
2. Objectives
The present study aimed at the neurobehavioral and histopathological evaluation of oral adenosine-enriched Japanese sake yeast in a mouse model of morphine withdrawal syndrome.
3. Methods
3.1. Animals
Male NMRI mice weighing 20 to 30 g were used for the present study. Standard conditions, including temperature, light/dark (LD), food, and water, were maintained the same for all the animals. The adaptation phase to the vivarium was exerted in animals 1 week before starting the experiments. All the experiments were performed in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and the local FUM Committee for Human and Animal Ethics.
3.2. Drugs
The dried sake yeast powder (GSP6), provided by Dr. Yuki Nagamori from Lion Corporation in Odawara-shi, Japan, was used. The powder was dispersed in the water shortly before use and then administered to the animals by oral gavage at doses of 100, 200, or 300 mg/kg. The sake yeast doses were chosen based on previously published studies (16). Methylcellulose, used as a vehicle for the sake of yeast, and 8‐cyclopentyltheophylline (CPT-8) were purchased from Sigma-Aldrich (St Louis, MO, USA). ZM241385 was purchased from Tocris Bioscience (Bristol, UK). Morphine sulfate was purchased from Darou Pakhsh Pharmaceutical MFG Co. (Tehran, Iran) and administered subcutaneously (s.c.). Diazepam was purchased from Sobhan Darou Pharmaceutical Co. (Tehran, Iran). Additionally, CPT-8 and ZM241385 were injected intraperitoneally. Sake, methylcellulose, and diazepam were administered orally.
3.3. Experimental Design and Pharmacological Phase
The mice were administered orally with sake yeast for a week and concurrently in the last 3 days of sake yeast administration. The animals were rendered dependent by gradually increasing doses of morphine sulfate (s.c. twice daily with 12-hour intervals for 3 consecutive days). Administered doses were 30 and 45 mg/kg on the first day, 60 and 90 mg/kg on the second day, and 90 mg/kg on the last day (17). After the continuous intraperitoneal (i.p.) injections of morphine, the withdrawal signs were observed and monitored following the two experimental protocols; firstly, it was by the i.p. administration of naloxone (3 mg/kg), 3 hours after the last dose of morphine (18). Immediately after the injection of naloxone, the animals were housed in a confined wooden box with high vertical walls. The number of jumps was counted, and the other signs, including chattering teeth, irritability, tremors, diarrhea, ptosis, piloerection, genital grooming, squirming posture, and wet dog shaking, were monitored over the 30-minute period (310). Each character was shown with a point given for 10 minutes (scoring range: 0 - 3) (17). Secondly, it was spontaneously, immediately after the sudden cessation of morphine, and the mentioned withdrawal signs were checked in mice for 24 hours after the last dose of morphine. Immediately after recording behavioral data, the brains were removed, dissected, and prepared for in vitro test.
A total of 100 mice (in general, 200 mice were used for both methods of inducing naloxone and spontaneous withdrawal syndrome) were randomly divided into 10 groups. The animals of all the following groups were exposed to 3-day increasing doses of morphine (in all groups equal to n = 10):
(1) Control group (CONT)
(2) Mice were orally administered (VEH) once daily with 0.5% methylcellulose in water (vehicle) for 1 week.
(3) Mice were orally administered diazepam (2.5 mg/kg) daily for 1 week (DZP).
(4), (5), and (6) Three treatment groups of mice were orally administered sake yeast powder in water (100, 200, and 300 mg/kg) (SAKE).
(7) Mice were orally administered 0.5% methylcellulose in water (vehicle) once daily for 1 week and finally injected with a single dose of 8-cyclopentyltheophylline (a selective adenosine A1R antagonist, 10 mg/kg, i.p.) on day 7 (VEH + CPT).
(8) Mice were orally administered sake yeast powder in water (200 mg/kg) once a day for 1 week and finally injected with a single dose of 8-cyclopentyltheophylline (10 mg/kg, i.p.) on day 7 (SAKE + CPT).
(9) Mice were orally administered 0.5% methylcellulose in water (vehicle) once daily for 1 week and finally injected with a single dose of ZM24138 (a selective adenosine A2AR antagonist, 15 mg/kg, i.p.) on day 7 (VEH + ZM).
(10) Mice were orally administered sake yeast powder in water (200 mg/kg) once a day for 1 week and finally injected with a single dose of ZM24138 (15 mg/kg, i.p.) (12).
3.4. Histopathological Evaluation
The histopathological changes in the hippocampal CA1 region were examined using Nissl staining. After recording behavioral data from spontaneous morphine-withdrawn animals, the mice were anesthetized using the i.p. administration of chloral hydrate (35 mg/mL) and then perfused with 0.9% saline solution plus 4% paraformaldehyde. Immediately, the brain tissue was dissected, removed, and postfixed at 4°C in 4% paraformaldehyde for 12 hours. The tissue was then placed in paraffin. The paraffin-embedded tissues were washed by running water, underwent graded ethanol dehydration, and then placed in paraffin again. Tissue samples were prepared for Nissl staining after sectioning (4 μm thick), deparaffinized in xylene, and rehydrated in ethanol (100 - 70%). The sections were washed with phosphate-buffered saline (PBS), then dried at 70°C for 2 hours, immersed in 0.9% cresyl violet (Sigma-Aldrich) for 1 hour at 56°C, rinsed with distilled water, differentiated in 95% alcohol, dehydrated with 70%, 80%, 90%, and 100% ethanol, cleared in xylene, and then mounted with neutral balsam. Eventually, the number of surviving pyramidal cells in the hippocampal CA1 region (per 1 mm length of each section) was counted in a blind manner using a microscope (× 400).
3.5. Data Analysis
GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA) was applied for statistical analysis. For counting jumps, the data were expressed as the mean ± standard error of the mean (SEM), and the statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by Tukey’s posthoc test. A P-value < 0.05 was considered a significant result. The Mann-Whitney U test was applied for the analysis of the qualitative withdrawal signs.
4. Results
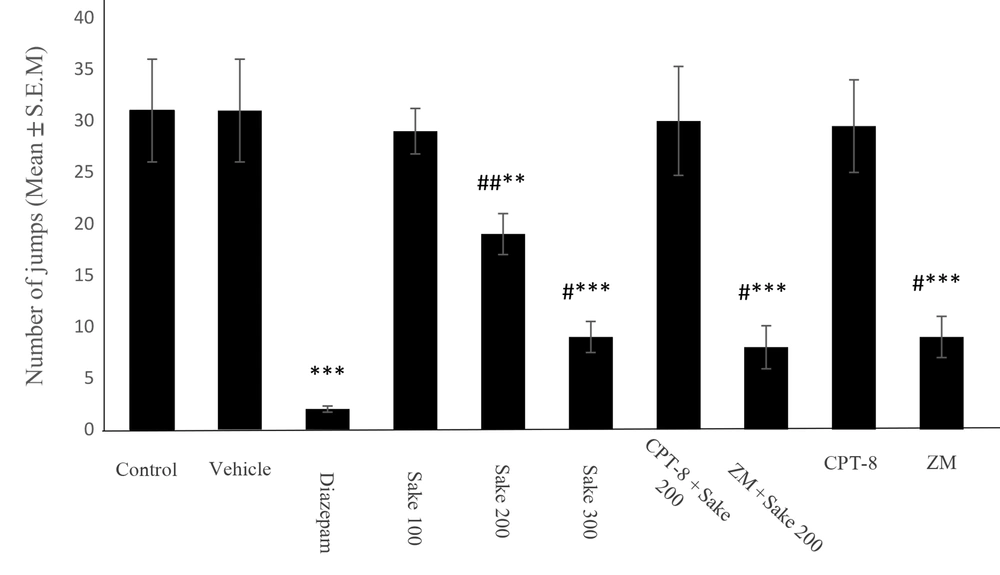
As shown in Table 1, morphine-treated mice demonstrated a significant increase in the number of jumping after the induction of withdrawal syndrome compared to the intact mice. As shown in Figure 1, similar to 2.5 mg/kg of diazepam (P < 0.001, compared to the control group), sake yeast led to a significant reduction of the number of jumps in naloxone-precipitated withdrawal syndrome in morphine-dependent mice (one-way ANOVA: F 3,36 = 22.83, P < 0.001). For 100, 200, and 300 mg/kg of supplement, this reduction was 6.45%, 38.7%, and 70.96%, with statistical significances reported as P > 0.05, P < 0.01, and P < 0.001, compared to the control, respectively. Moreover, 100 mg/kg of supplement did not show a significant difference compared to the control.
| Groups/Parameters | Weight (g) | Number of Jumping |
|---|---|---|
| Intact | 23 ± 3.22 | 0 |
| Morphine-treated mice and withdrawn by naloxone | 23.4 ± 3.1 | 19 ± 2.34 ** |
| Morphine-treated mice and withdrawn spontaneously | 22.8 ± 2.61 | 3 ± 0.74 * |
a The data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences are reported from the intact mice (*P < 0.05, * *P < 0.01).
b In all groups, n = 10.
Effect of sake yeast (mg/kg) on the number of jumps during the naloxone-precipitated morphine withdrawal in mice. The data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences are reported from the control group (** P < 0.01, ***P < 0.001) or from the diazepam group (#P < 0.05, ##P < 0.01). In all groups, n = 10, CPT-8: 8‐cyclopentyltheophylline; ZM: ZM241385
Additionally, 100 mg/kg of sake yeast significantly decreased the intensity of naloxone-precipitated tremor compared to the control group (U-value = 25, P < 0.05). The intensity attenuation of irritability (agitation, U-value = 25), tremor (U-value = 5), diarrhea (U-value = 5), ptosis (U-value = 5), piloerection (U-value = 5), genital grooming (U-value = 0), writhing posture (U-value = 0), and the wet dog shake (U-value = 0) was observed with the administration of 200 mg/kg of sake yeast (P < 0.05), and all of the mentioned withdrawal signs (except for teeth chattering, U-value = 50) were attenuated with 300 mg/kg of the supplement (P < 0.05) (Table 2). Teeth chattering did not show any significant difference between the control and all the sake yeast-treated (lonely) groups (P > 0.05).
| Groups/Parameters | Teeth Chattering | Ptosis | Piloerection | Diarrhea | Irritability | Tremor | Genital Grooming | Writhing Posture | Wet Dog Shake |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 3 (0 - 3) | 3 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) |
| Vehicle | 1 (0 - 3) | 3 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 3 (0 - 3) | 2 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) |
| Diazepam | 0 (0 - 3) * | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 1 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) * | 0 (0 - 3) * | 0 (0 - 3) * |
| Sake 100 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 3 (0 - 3) | 2 (0 - 3) * | 1 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) |
| Sake 200 | 1 (0 - 3) | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 2 (0 - 3) * | 1 (0 - 3) ** | 0 (0 - 3) * | 0 (0 - 3) * | 0 (0 - 3) * |
| Sake 300 | 1 (0 - 3) | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 1 (0 - 3) ** | 0 (0 - 3) * | 0 (0 - 3) * | 0 (0 - 3) * |
| CPT-8 + Sake 200 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) * | 3 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) |
| ZM + Sake 200 | 0 (0 - 3) * | 1 (0 - 3) * | 0 (0 - 3) ** | 1 (0 - 3) * | 1 (0 - 3) ** | 1 (0 - 3) ** | 0 (0 - 3) * | 0 (0 - 3) * | 0 (0 - 3) * |
| CPT-8 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 3 (0 - 3) | 2 (0 - 3) * | 1 (0 - 3) | 1 (0 - 3) | 1 (0 - 3) |
| ZM | 0 (0 - 3) * | 0 (0 - 3) ** | 0 (0 - 3) ** | 1 (0 - 3) * | 1 (0 - 3) ** | 1 (0 - 3) ** | 0 (0 - 3) * | 1 (0 - 3) | 0 (0 - 3) * |
a Withdrawal signs were monitored over 3 × 10 min periods, and the scores were given to the animals (0 - 3). The data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences are reported from the control group (*P < 0.05, **P < 0.01) using the Mann-Whitney U test.
b In all groups, n = 10.
c CPT-8: 8‐cyclopentyltheophylline; ZM: ZM241385
In this study, 100 mg/kg of sake yeast showed no significant differences in spontaneous-precipitated withdrawal signs compared to the control group (P > 0.05). The intensity attenuation of irritability (U-value = 0), tremor (U-value = 0), diarrhea (U-value = 0), ptosis (U-value = 0), and piloerection (U-value = 0) was observed by 200 mg/kg (P < 0.05) or 300 mg/kg (P < 0.01) of sake yeast (Table 3). Teeth chattering, genital grooming, writhing posture, and wet dog shake showed no significant difference between the control and the sake yeast-treated groups (P > 0.05). Furthermore, the withdrawal symptoms-attenuating effect of sake yeast was inhibited by the administration of CPT-8 (lonely or in combination with 200 mg/kg of supplement) and maintained following the administration of ZM241385 (Figure 1, Tables 2, and 3).
| Groups/Parameters | Teeth Chattering | Ptosis | Piloerection | Diarrhea | Irritability | Tremor | Genital Grooming | Writhing Posture | Wet Dog Shake |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| Vehicle | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| Diazepam | 0 (0 - 3) * | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| Sake 100 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| Sake 200 | 1 (0 - 3) | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| Sake 300 | 1 (0 - 3) | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) ** | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| CPT-8 + Sake 200 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| ZM + Sake 200 | 1 (0 - 3) | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| CPT-8 | 1 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 2 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3) |
| ZM | 1 (0 - 3) | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 1 (0 - 3) * | 0 (0 - 3) | 0 (0 - 3) | 0 (0 - 3 |
a The signs were evaluated 24 hours after the last dose of morphine, and the scores were given to the animals (0 - 3). The data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences are reported from the control group (*P < 0.05, * *P < 0.01) using the Mann-Whitney U test.
b In all groups, n = 10.
c CPT-8: 8‐cyclopentyltheophylline; ZM: ZM241385
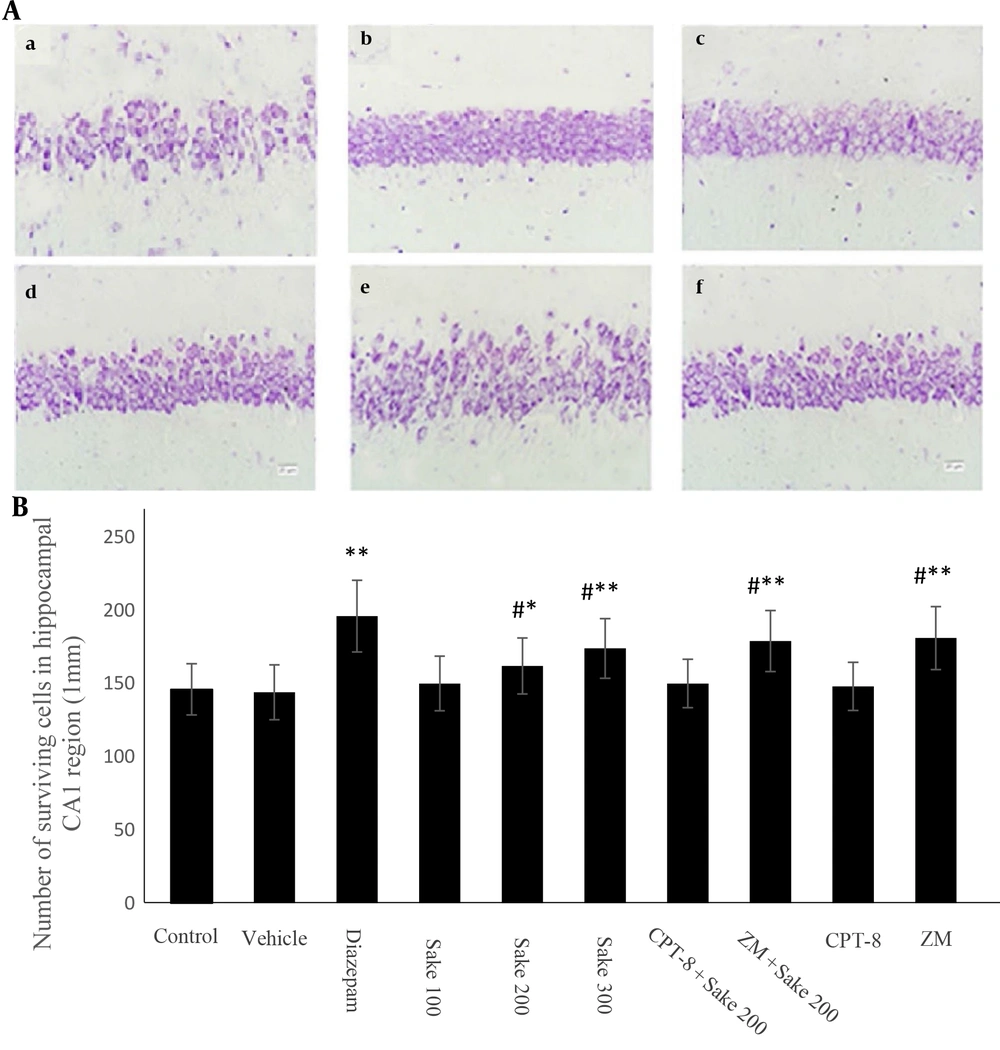
In the diazepam group (i.e., diazepam-treated morphine-withdrawn mice), the neurons in the hippocampal CA1 region were demonstrated round-shaped, and cytoplasmic intact cells were shown without any distorted morphology. In the control group (i.e., non-treated morphine-withdrawn mice), masses of dysplastic neurons were scattered; disappearance, irregularity, swelling, and cracking of the neurons were obvious, and the number of Nissl bodies was diminished in the cytoplasm and nuclear pyknosis. The aforementioned signs were attenuated in mice treated with 200 and 300 mg/kg of sake yeast. The number of surviving cells was quantitatively analyzed in each group and is statistically shown in the histogram (Figure 2). The number of surviving neurons increased significantly in the diazepam group (P < 0.01) and by 200 and 300 mg/kg of sake yeast (P < 0.05) compared to the control group. Similar to the previously mentioned results, these effects were also inhibited by the administration of CPT-8 (in combination with 200 mg/kg of the supplement) and maintained following the administration of ZM241385.
Nissl staining of the hippocampal CA1 (A) pyramidal neurons with cresyl violet (× 400 Magnification); (a), control; (b), diazepam; (c), sake 200; (d), sake 300; (e), CPT-8 + sake 200; (f), ZM + sake 200; Bar 20 μm; quantitative analysis of the expression of surviving neurons in the hippocampal CA1 (B). The data were expressed as mean ± standard error of the mean (SEM). Statistically significant differences are reported from the control group (* P < 0.05, **P < 0.01) or from the diazepam group (#P < 0.05). In all groups, n = 10. CPT-8: 8‐cyclopentyltheophylline; ZM: ZM241385
5. Discussion
Adenosine receptors and their functions have been shown to be adjusted by chronic opiate treatment (19, 20). The result of the present study revealed that sake yeast supplement (enriched by high concentrations of oral adenosine analogs) exerts neuroprotection, including behavioral and morphological improvement, in spontaneous and naloxone-induced morphine withdrawal syndrome. Reversing and maintaining this effect following CPT-8 and ZM241385 injections, respectively, demonstrated that sake yeast exerted a withdrawal symptoms-lowering effect predominantly by the activation of A1Rs that is consistent with the relevant previous findings; the adenosine A1R selective agonists, N6-cyclohexyladenosine (CHA) and N6-phenylisopropyladenosine decrease jumping and diarrhea in naloxone-precipitated morphine withdrawal symptoms in mice (19). Subsequently, jumping increased; nevertheless, diarrhea decreased with the administration of the adenosine A1R antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX).
The adenosine A2R agonist, 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA), decreases jumping and diarrhea, and another adenosine A2R antagonist, 3,7-dimethyl-1-propargylxanthine (DMPX), did not elicit any response in this respect. 8-Cyclopentyl-1,3-dipropylxanthine decreases the inhibition of jumping and diarrhea induced by CHA; however, DMPX decreases the inhibition of diarrhea induced by CPCA (19). The aforementioned findings indicate that jumping induced by naloxone in morphine-dependent mice might be modified by the adenosine A1R mechanisms, and diarrhea induced by the opioid receptor antagonist could be mediated by both adenosine A1Rs and A2Rs. The local administration of a selective adenosine A1R agonist, N6-cyclopentyladenosine (CPA), into the nucleus accumbens decreased the Gellert-Holtzman withdrawal scale and attenuated opioid withdrawal symptoms in a dose-dependent manner (21). A2ARs are required to induce quasi-morphine withdrawal syndrome by the co-administration of caffeine and naloxone (22). The adenosine A1R agonist R-N6(phenylisopropyl) adenosine significantly reduces wet dog shakes and diarrhea; nonetheless, the adenosine A1R antagonist DPCPX significantly increases weight loss (19).
The inhibition of the N-type calcium channels via the opioid receptors is considered the underlying mechanism of morphine (23). The long-term consumption of opioids, such as morphine, leads to a decrease in calcium influx into the neurons; following that, the release of glutamate would be diminished, and its NMDA receptor would be upregulated. When morphine consumption is ceased, the calcium channels change their status to the open state. The excessive calcium influx releases a large amount of the main excitatory neurotransmitter (glutamate). Eventually, neuronal cell death occurs and causes a withdrawal syndrome (24, 25).
On the other hand, the neurological effects of sake can be induced by its adenosine analogs. Adenosine, known as a neuromodulator, has biphasic excitatory and inhibitory effects on neurotransmission, acting via the inhibitory adenosine A1R and the less common facilitating A2AR. It is well known that adenosine receptors play an important role in neuroprotection as they inhibit glutamate release and hyperpolarize neurons (26). Adenosine mainly inhibits intraamygdalar circuits through A1R activation. The activation of A1R by the selective A1R agonist CPA decreased NMDAR-mediated currents, and the activation of A2AR with a selective A2AR agonist, CGS21680 ([2-p-[2 carboxyethyl] phenethylamino-5¢-N-ethylcarboxamidoadenosine hydrochloride hydrate [CGS21680]) also decreased NMDAR-mediated synaptic currents in the amygdala. This effect inhibited by the A1R antagonist, DPCPX, rather than by the selective A2AR antagonist, 2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e] [1,2,4] triazolo[1,5-c]pyrimidin-5-amine (SCH58261) (27). Therefore, sake yeast-induced neurobehavioral protection can be attributed to activating the adenosine receptors (mainly A1Rs) and inhibiting glutamatergic transmission in a withdrawal syndrome.
5.1. Conclusions
Eventually, this study concluded that both adenosine A1R and A2AR are involved in opioid dependency and withdrawal syndrome. The activation of A1Rs and blockade of A2ARs elicit a withdrawal syndrome-attenuating effect. Oral adenosine-enriched sake yeast appears to primarily stimulate A1Rs. The key benefit of using Japanese sake yeast and its active component SAM, compared to injectable synthetic adenosine and adenosine A1R agonists, is the availability of non-invasive oral forms, such as powders or tablets. Additionally, this natural compound might have lower drug resistance and improved tolerance in patients.
Further research is needed to determine the optimal dosage of sake yeast that provides maximum neurobehavioral protection with minimal CNS depression. Although the authors of this study previously reported the memory-enhancing (28), anxiolytic, and antidepressant-like effects (29) of sake yeast, the present preclinical data provide basic evidence for the clinical use of sake yeast in patients suffering from morphine withdrawal syndrome. Therefore, additional physical activity (30), the stimulation of adenosine receptors by natural compounds, such as sake yeast, would be useful for neurobehavioral problems.