1. Background
Denture stomatitis (DS), also known as denture sore mouth and stomatitis prosthetica, is a common condition in patients using dentures with poor oral hygiene (1). Denture stomatitis is characterized by a patch or generalized erythema in the oral mucosa covered by dentures, and it is more common in women maxillas (2, 3). Any discomfort or clinical symptoms are less likely to occur in DS cases. With 11 to 72% prevalence, DS is the most common problem among denture users (1, 2). An ill-fit denture can lead to mucosal trauma and increases the risk of adhesion and growth of variant microorganisms like Candida species (4).
In older studies, systemic disorders, especially immunodeficiency, were introduced as the main factors for DS (5, 6). However, in recent studies, local and systemic measures like trauma, Candida infections, decreased saliva, some medications, poor oral hygiene, diet, and ill-fitting dentures have been proposed as the contributing factors for DS (4, 6).
Although the causes of DS are multifaceted, the most common factor in its development is the Candida albicans biofilm (7). Systemic antifungal drugs such as nystatin are effective in people with acute candidiasis. Still, nystatin cannot completely control the infection with predisposing factors such as a denture, and recurrences are common (8, 9). Studies have shown that Candida biofilm can attach to the surface of oral mucosa and acrylic resins. It can grow in the cracks and pores of the denture surface, which will be protected from common antifungal and antibiotic agents. Evaluation of pathogenic microorganisms on the surface of old and new dentures showed that more than 85% of old and 50% of new dentures contain pathogenic microorganisms. Since regular denture changes are not possible and affordable for most people, finding ways to control biofilm formation is essential (1-6, 10).
Nanomaterials are made of particles with at least one diameter of fewer than 100 nanometers (nm). These materials have various medical and industrial applications (11-15). Silver nanoparticles (AgNPs) have a variety of antimicrobial activities. They are reportedly capable of killing and reducing the colony number of a wide variety of gram-positive and gram-negative bacteria like Clostridium perfringens, Streptococcus mutans, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa (13-16). The antibacterial mechanisms of AgNPs have not been properly understood yet. However, disruption of the bacterial cell wall and cytoplasmic membrane due to silver ions (Ag+) release, denaturation of ribosomes, interruption of adenosine triphosphate (ATP) production, and interference with deoxyribonucleic acid (DNA) replication are some of the suggested mechanisms (17).
Some antifungal applications are also reported for AgNPs (15, 17). These particles seem to have a fungicide activity against some Candida sp. like C. nonalbicans and C. tropicalis (18). A review article conducted by Rozhin et al. (18) found that these antifungal activities were correlated to the concentrations of nanoparticles, not the release of silver ions.
Various articles analyzed the effects of AgNPs on Candida sp. (19-23). However, no study has been conducted to analyze the effects of these nanoparticles on the nystatin-resistant species. Only Falcao et al. (24) evaluated AgNPs’ impacts on fluconazole-resistant Candida albicans strains. Considering the high frequency of Candida infections and an increasing number of drug-resistant species, exploring new approaches to controlling and treating such infections seems inevitable.
2. Objectives
Considering the increased nystatin-resistant strains of Candida, their problematic infections, and the lack of a guaranteed treatment procedure in such cases, this study aimed to evaluate the minimum growth inhibitory concentration (MIC) of AgNPs on nystatin-resistant C. albicans fungal biofilms in DS patients.
3. Methods
This study’s methodology was approved by the ethical committee under the ethical number of IR.IAU.DENTAL.REC.1399.255.
3.1. Materials
Potassium hydroxide (KOH) was purchased from Ghatran Shimi Tajhiz (Tehran, Iran). Sabouraud dextrose agar culture medium (SDA), Sabouraud dextrose broth culture medium (SDB), and the glycol-based AgNPs with a size of 10 - 20 nm and 400 µg/mL concentration were purchased from Merck (Germany). Phosphate-buffered saline (PBS), RPMI1640 medium, and MTT were purchased from Sigma-Aldrich (Munich, Germany). CHROMagar Candida was purchased from Taligene (Isfahan, Iran).
3.2. Method
This study analyzed 21 C. albicans strains (20 oral C. albicans strains and 1 standard C. albicans; ATCC:10261). The specimens were isolated from the inner surface of the patient’s dentures with DS who had been studied in previous projects, and their samples were available in the Department of Medical Parasitology and Mycology repository. After biofilm formation, all samples were exposed to nystatin, and the nystatin-resistant specimens were selected for second biofilm formation and AgNP exposure. Silver nanoparticles with a size of 10 - 20 nm and 400 µg/mL concentration were used in the present study. The test was repeated three times for each specimen, and the sampling method was random.
3.2.1. Identification of Candida albicans Strains
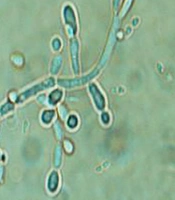
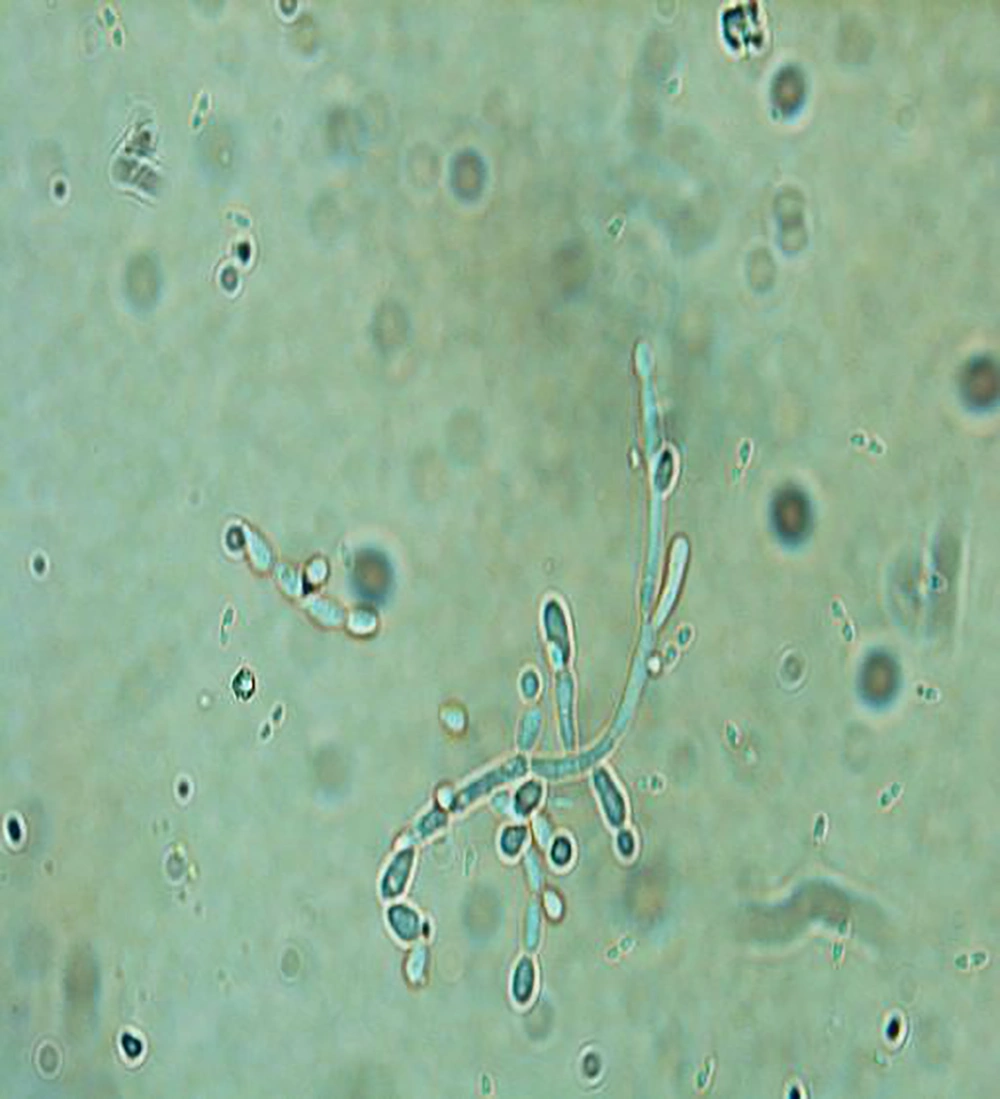
All specimens were stored in a laboratory tube containing 0.5 milliliters (mL) of distilled water at the mycology laboratory at the temperature of -70 degree Celsius (°C). The species were recovered then. After the preparation of their smears, they were stained with 10% KOH solution. After that, the presence of budded or non-budded yeast cells and pseudohyphae was confirmed using a microscope (Figure 1).
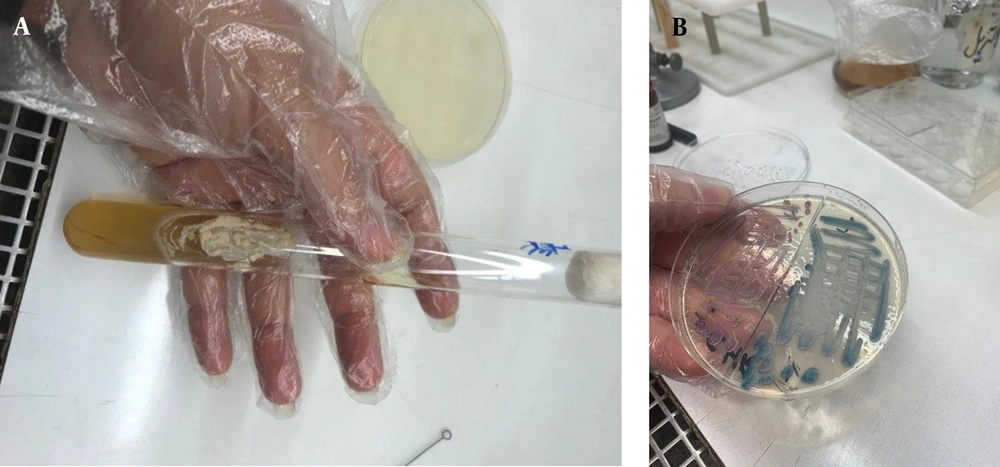
Meanwhile, a sterile swab was placed on the SDA culture medium and incubated at 37°C for 12 to 24 hours (hrs) to form cream-colored yeasts (Figure 2A). These yeasts, then, were placed on Candida chromagar coloring (CAC) medium and incubated at 37°C for 12 hrs. After incubation, 20 strains of C. albicans were identified with the produced green color (25) (Figure 2B).
3.2.2. Biofilm Preparation
The specimens were cultivated on the SDA culture medium for yeast biofilm preparation and incubated for 24 - 48 hrs. Then, a loop of grown specimens was inoculated into 5 mL SDB medium and incubated for 24 hrs before 10-minute (min) centrifuging at 3000 rpm. After removing the supernatant, 5 mL of 1X PBS was added to the tube and centrifuged at 3000 rpm for another 10 min. The remaining cells and RPMI1640 medium were used to prepare a million per milliliter concentration of yeast cells using a spectrophotometer device (Apple, Japan). After that, 100 microliters (µL) of the prepared solution were poured into each microplate well and incubated for 24 hrs at 37°C. Then, the solutions in the wells were washed twice with PBS. After each washing step, the solutions were removed, and the microplate was placed on sterile gauze or tissue to dry. In the end, 100 µL of RPMI culture medium was added to all microplate wells, and 100 µL nystatin was added to row A. After a complete up and down of solutions using a sampler, 100 µL was removed from the first well containing the drug and culture medium and added to the second well, followed by removing 100 µL from the second well and adding it to the third well. This process was repeated for the 10th well and in the second and third rows of the microplate. In each row, the last 100 µL was discarded. The 11th well was a positive control containing culture medium and yeast suspension, and the 12th was a negative control with only culture medium. In the end, the microplate was closed and incubated for 24 hrs before evaluation of MIC.
This experiment was repeated three times. A microscopic slide was prepared from the highest MIC dilution to confirm the MIC results, and the growth or non-growth of C. albicans yeasts was observed using a microscope.
3.2.3. MIC Assessment
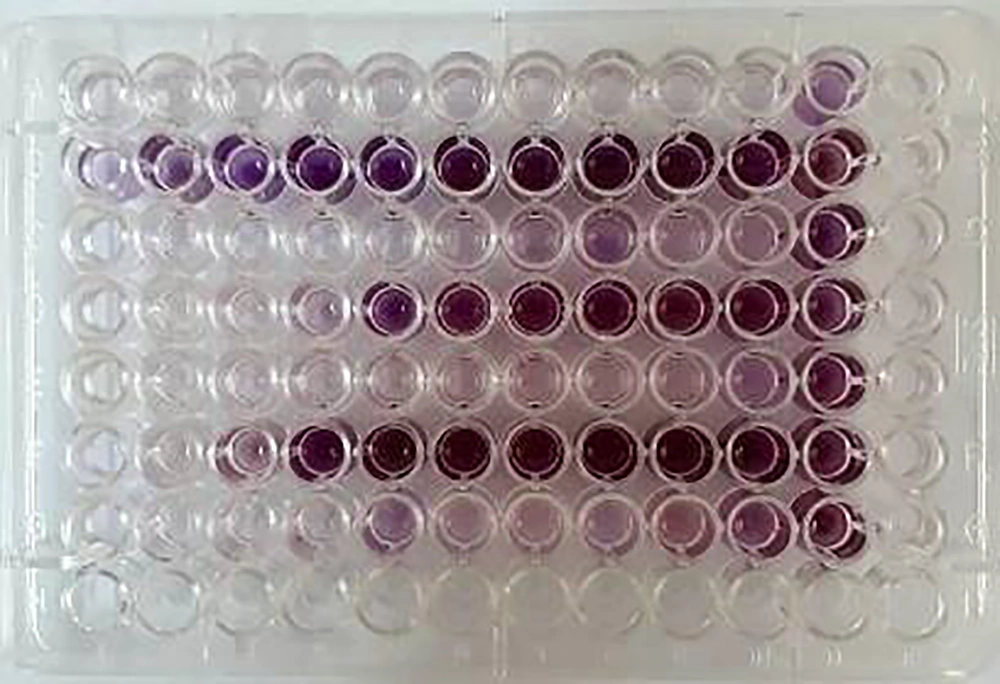
The MIC assessment was performed using MTT. 5 mg of MTT powder was dissolved in 1 mL PBS and diluted using 9 mL RPMI1640. The biofilm was prepared in the microplate for the second time, and 100 µL nystatin was added to the first well of row A, followed by the serial dilution of nystatin. 50 μL MTT solution was added to the wells, and the microplates were incubated in a dark place for 2 - 3 hrs. After that, the supernatant solution was aspirated, and 150 mL of dimethyl sulfoxide (DMSO) solution was added to the wells. 100 µL of the solution was added to a new microplate as a blank. After the color change (from yellow to purple) (Figure 3), the absorbance was measured at 570 nm using an ELISA reader (Model 550, BioRad). Based on the obtained optical density (OD) compared with the blank OD and The Clinical & Laboratory Standards Institute (CLSI) standard protocol, MIC was assessed. Species with a MIC of more than 1 were categorized as resistant species (26).
The nystatin resistance specimens formed biofilms and were exposed to 400 µg/mL concentration of AgNPs. Then, the MIC assessment was performed and compared with the nystatin group.
3.3. Statistical Analysis
The normal distribution of data was analyzed using the Kolmogorov-Smirnov test, and the comparison between the two examined groups was performed using Mann-Whitney. The significant level was 0.05.
4. Results
In this study, 20 oral Candida specimens were evaluated. The results revealed that 8 specimens (40%) represented a MIC of more than 1 when they were exposed to nystatin. These specimens were exposed to AgNPs, and their MIC was reported. The results revealed that AgNPs were effective against 6 resistant species (75% of all resistant specimens) and could reduce MIC up to 2 units (Table 1).
| No. | MIC (µg/mL) | |
|---|---|---|
| Nystatin | Nanosilver | |
| 1 | 2 | 0.5 |
| 2 | 4 | 2 |
| 3 | 2 | 1 |
| 4 | 0.5 | - |
| 5 | 0.25 | - |
| 6 | 0.5 | - |
| 7 | 0.5 | - |
| 8 | 2 | 2 |
| 9 | 1 | - |
| 10 | 1 | - |
| 11 | 2 | 2 |
| 12 | 0.5 | - |
| 13 | 0.5 | - |
| 14 | 4 | 1 |
| 15 | 1 | - |
| 16 | 4 | 1 |
| 17 | 2 | 0.5 |
| 18 | 0.5 | - |
| 19 | 0.25 | - |
| 20 | 1 | - |
| C. albicans (ATCC10261) | 0.5 | 0.25 |
Minimum Growth Inhibitory Concentration Results of Candida albicans in Exposure to Nystatin and Silver Nanoparticles
The results of the Kolmogorov-Smirnov test did not confirm the normality of data distribution (P = 0.001). So, the non-parametric tests were applied for data analysis.
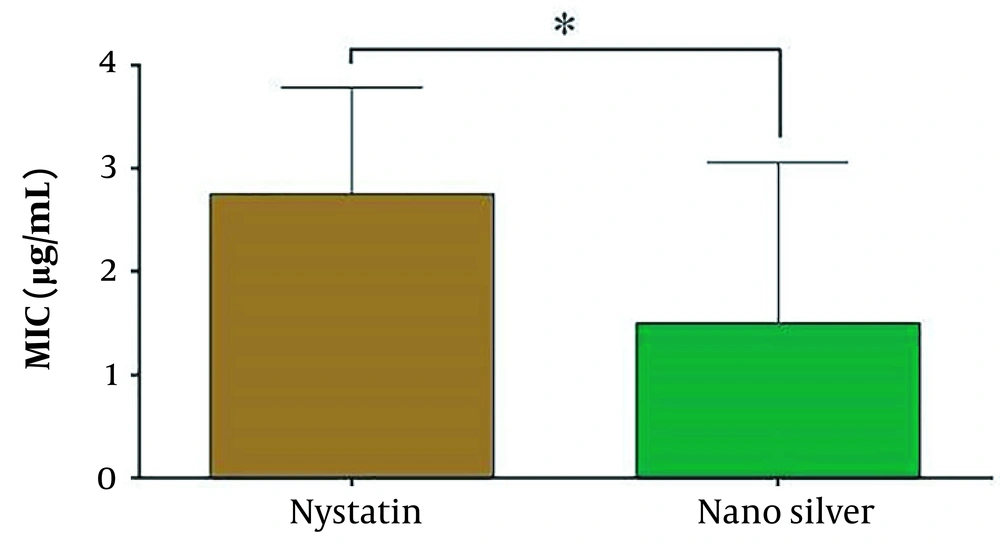
Figure 4 shows the efficacy of nystatin and AgNPs on C. albicans in the bar charts.
Comparing the two groups using the Mann-Whitney test showed a significant difference between the application of nystatin and AgNPs. The AgNPs showed more effectiveness than nystatin against C. albicans (P = 0.038).
5. Discussion
The oral cavity is the habitat of more than 300 different microorganisms, and the presence of pathogenic species can increase due to any interventions in this environment. Dentures are one of the most common prosthetic appliances used to treat edentulous patients, replace their natural teeth, and restore their function and beauty. Despite all the merits of using dentures, they can also lead to DS, one of the most common inflammations in edentulous patients (4, 11, 27, 28).
Candida albicans is the most reported DS-related microorganism (9, 28-31). Unfortunately, many Candida biofilms are resistant to common antifungal drugs like fluconazole, nystatin, and amphotericin B, and frequent recurrences have been observed (8, 32, 33). The present study shows that 8 oral Candida sp. (40%) resisted the typical nystatin therapy. Due to the critical role of the contact surface’s physical and chemical structure in biofilm formation, many studies have been conducted on preventing components (11, 12, 34, 35). Some of these components are prescribed with antifungal drugs for higher efficiency. Cyclooxygenase inhibitors (aspirin and diclofenac) are the first examples that can eliminate C. albicans biofilm formation by reducing prostaglandin production. Calcineurin inhibitors (such as cyclosporin A and tacrolimus (FK506) are the other examples prescribed with amphotericin B and fluconazole. In the presence of calcineurin inhibitors, the synergistic effect leads to changes in the fungistatic effects of fluconazole into a fungicide. In addition, by destroying the membrane, azoles increase the drug concentration inside the cells (36). Despite all these components, still, Candida sp. can resist and infect vulnerable people (37).
Nanotechnology is a new technology that makes it possible to design nano-scale materials without changing their main properties. Thanks to this technology, the methods of infection control have developed dramatically. Nanomaterial-covered equipment used in medicine is an example that reduces infection transmission and the prevalence of nosocomial infections (9, 11, 12, 35, 38).
Silver nanoparticles showed antifungal properties in some studies. Li et al. revealed that AgNPs are helpful in treating local infections caused by C. albicans and Candida tropicalis. He believed AgNPs could increase topical antifungal activities by combining carbon nanoscroll and graphene oxide (39). Monteiro et al. found that AgNPs combined with nystatin and chlorhexidine have synergistic anti-biofilm effects, depending on the species type and the drug concentration (40). Silva et al. showed that both AgNPs and nystatin could be effective on either mixed or solo C. albicans and Glabrata fungi biofilms grew on acrylic resins. However, this effect was more significant for AgNPs (41). Like the present study, Nozari et al. showed more efficiency of AgNPs than nystatin in her study (38). Kim et al. also found a higher impact of NPs on MIC and its significant reduction compared to nystatin, similar to the results of our study (42). Nozari et al. stated that the introduction of AgNPs in pharmaceutical formulations of nystatin can be helpful in the treatment of candidiasis. Although 16 - 128 μg/mL nystatin can inhibit Candida sp. growth, its antifungal activities can significantly improve with AgNPs (38).
In the present study, nystatin-resistant specimens were exposed to AgNPs, which stands out among all the mentioned studies. Drug-resistant microorganisms can be hazardous, especially for high-risk groups. Considering the prevalence of C. albicans and its fatal infections in vulnerable patients, finding more reliable treatment procedures to combat the resistant strains is vital. Nosocomial infections associated with C. albicans are the fourth leading cause of this type of infection. Other than the resistance of Candida sp. to the common antifungal drugs, the constant consumption of these drugs can lead to hepatotoxicity and nephrotoxicity (37).
Comparing nystatin, the results of this study showed considerably more effectiveness for AgNPs regarding MIC reduction. Few reported side effects for AgNPs are available, which refer to their adverse effect on the gut microbiome (13). Since, in the case of oral candidiasis and Candida-associated DS, applying topical medications is possible, there is less concern about these side effects. It seems that the benefits of AgNPs outweigh their drawbacks, especially in oral drug-resistant species.
5.1. Conclusions
According to the results of this study, it can be concluded that both nystatin and AgNPs are effective against Candida biofilms formed in the denture surfaces. However, it seems that the efficiency of AgNPs is significantly higher. Considering the few reported complications for AgNPs, their application for treating DS patients with Candida biofilms can be a practical approach, although more in vivo and trial studies need to be conducted to clarify the infinite positive and negative effects of these AgNPs.