1. Context
In most communities throughout the world, cancer is the leading or second leading cause of early death. It is predicted that cancer patients worldwide are projected to increase significantly during the next 50 years due to demographic changes, such as population aging. Cancer rates are expected to rise in all socioeconomic nations throughout the next 50 years, while the incidence is greater in countries with a low Human Development Index (HDI). A doubling in the incidence of all cancers is considered by 2070 compared to 2020 if the major cancer types' incidence patterns remain the same (1). The highest increase in cancer incidence is expected in Low- to Middle-income Countries (LMICs) because of the rapid changes in socioeconomic conditions (2).
There are generally no indications or symptoms in the early stages of cancer development. The growth of the masses may be painless at times. Still, the individual may experience discomfort when cancer has progressed to an advanced stage, which increases the early mortality of the patients. As a result, cancer treatment delays are a serious issue in healthcare systems worldwide (3). Traditional cancer treatment is based on targeting high proliferative cells. Depending on the type and stage of cancer, the treatment options can be chemotherapy, surgery, radiation therapy, hormonal therapy, and targeted therapy. Besides, combination therapy can increase the effectiveness of cancer treatment. The disadvantage of chemotherapy and radiotherapy is that they can damage healthy tissues. Cancer treatment aims to achieve complete remission, and if that is not possible, it aims at reducing the severity of the cancer growth to reduce the patient's symptoms and improve their quality of life. In the recent decade, our knowledge about the biology and signaling of cancer has advanced. Thus, the success and effectiveness of the treatments improved, which led to an increase in survival and life quality (4).
Targeted therapy mostly includes monoclonal antibodies and small molecule inhibitors, which are used to treat numerous cancers, like breast, colorectal, lung, and pancreatic cancers, lymphoma, leukemias, and multiple myeloma. Although targeted treatments are often more well-tolerated than regular chemotherapy, they are linked to several side effects: Acneiform rash, heart dysfunction, thrombosis, hypertension, and proteinuria (5). Myeloid ecotropic viral integration site (MEIS) proteins are members of the three amino acid loop extension (TALE) homeodomain transcription factor family. They play an important role in cell proliferation, differentiation, and apoptosis, which are crucial for growth and tissue regeneration (6). In addition, the MEIS family of homeodomain proteins helps enhance the transcriptional efficiency of Homeobox (HOX) transcription factors, which are crucial regulators of cellular processes and stem cell development. Consequently, any defects in these processes could result in growth defects and tumorigenesis (7).
Thus, nowadays, this family has turned into an interesting topic for oncology research and in the field of targeted therapy. According to research, each MEIS member may have carcinogenic or tumor-suppressive effects in different neoplasms. Thus, this uncertainty suggests that MEIS members have a unique function in cell proliferation and differentiation (8). Meng et al. discovered the expression level of MEIS family members in 33 cancer types. All three MEIS family genes were shown to be overexpressed in malignancies. There was much variation in the expression levels of each MEIS family member. The expression level of a certain gene was extremely high in some tumor types, whereas it was greatly down-regulated or remained unchanged in others. So, being a risk factor or a protective factor depends on the kind of cancer and even the subtype of a certain cancer (9). New research should evaluate MEIS members' roles in tumor development, cancer drug resistance, and immune response according to the type of cancer, and more studies should be conducted to know more about this family. This review will summarize recent findings of the MEIS protein family in some cancers, providing new insights into this field of study.
2. MEIS Proteins Function
The MEIS proteins, originally described as the "helper groups" of HOX proteins, are now emerging as important and highly diverse regulators of cellular behavior. In embryonic growth or tissue homeostasis by adult stem cells, MEIS agents can absorb a wide range of epigenetic modulators into chromatin. In addition, MEIS gene activity is fully regulated at various levels, from RNA expression, binding, and stability to post-translational protein modification and controlled intracellular availability, all of which respond to extracellular factors. It suggests the intriguing possibility that this deeply preserved protein family evolved to coordinate the interaction between chromatin, chromatin-modifying enzymes, and the temporal, spatial, or cellular requirements of the cell. The MEIS proteins may act as sensors of extracellular signals and quickly translate this information into a transcription result (10).
Studies have shown that MEIS proteins and their cofactors are misregulated in various cancers. These studies have shown that MEIS proteins can be diagnostic and therapeutic biomarkers for cancer and other related diseases. The HOX genes are transcription factors characterized by the presence of a domain of 60 protected amino acids attached to DNA called homeodomain (HD). These genes are involved in regulating cellular fate, growth, tumorigenesis, and stem cell function (11). The expression of the HOX genes is regulated by nuclear dynamics, transcriptional regulation, long noncoding RNAs (lncRNAs), RNA processing, miRNAs, and translational events. Amino acid loop extension 3 (TALE) proteins are one of the major HD proteins with 27 members, including MEIS1-3, Pbx1-4, Irx1-6, Mkx, Pknox1-2, Tgif1-2, and their pseudogenes (12).
There are three isoforms of MEIS1-3 in the mammalian body. The MEIS, HOX, and PBX proteins attach to DNA as a heterodimer or trimeric structure, increasing its half-life. The MEIS protein and PBX regulatory protein-1 (PREP1) also functionally compete with each other (13). According to the study, the deletion of MEIS1 reduces the expression of p21, p15, p16, and p19arf in cardiomyocytes, improves cell cycle function, and activates caspase-dependent apoptosis in some cells if it increases the expression of MEIS proteins. In contrast, the overexpression of MEIS1 and HOXA9 inhibits apoptosis and protects cells from the effects of apoptosis-inducing factors (14).
PBX-MEIS1 proteins are required to induce caspase-3 and caspase-8-dependent apoptosis. There are different isoforms of MEIS in different tissues and cells, which proves that MEIS can have specific cellular consequences. Somers et al. showed that the depletion of MEIS1, HOXA9, c-Myc, and Bcl2 was associated with caspase-dependent apoptosis. Also, MiR-155 regulation, the main stimulus of the MEIS pathway, leads to caspase-dependent apoptosis, which involves signaling the N-terminal c-Jun kinase in Acute Myeloid Leukemia (AML) (15). Studies have also shown that the MEIS1 protein is an important regulatory protein in the differentiation of human pluripotent stem cells (hPSCs) into functional hematopoietic cells (16). As known, MEIS1 suppresses the production of Reactive Oxygen Species (ROS) and targets hypoxia-inducing factors HIF-1α and HIF-2α, leading to scavenging in hematopoietic stem cells (17).
The MEIS-PBX heterotrimer is involved in neurogenesis processes. The formation of the MEIS2 heterodimer with PAX6 and DLX2 can proliferate periplomerular Dopaminergic neurons in the olfactory bulb (18). The MEIS2-PBX1 heterodimer can also alter chromatin structure and modulate gene activity. Trimmers formed by MEIS1, HOXA10, and PBX2 modulate the expression of target genes in the human endometrium (19). The cyclic adenosine monophosphate (cAMP)-responsive sequence (CRS1) in the P450 family 17 (CYP17) gene is a transcriptional regulatory factor that has sites for PBX binding and MEIS1. Also, PBX1 and MEIS1 bind to CRS1 to regulate cAMP-dependent transcription (20). The PBX-regulating protein (PREP1) controls MEIS1 expression through post-transcriptional regulation. As shown, PREP1 inhibits the interaction between PBX1 and MEIS1 in mouse embryonic fibroblasts, destabilizing MEIS1, and interaction between MEIS1 and DEAD-box blocks helicase 3 x-linked (DDX3x) and DDX5. Finally, it reduces MEIS1 tumorigenesis (21).
Dimerization with PBX3 stabilizes MEIS1 and allows it to regulate target genes, such as the FMS-associated tyrosine kinase 3 (FLT3) receptor and trivalent pseudokinase 2 (TRIB2), thereby increasing HOX9-mediated transfection. The MEIS1 protein, which does not bind to PBX3, is prone to ubiquitination and subsequent degradation. Mutations in the PBX binding region in MEIS1 can also prevent MEIS1 ubiquitination, as PBX3 and responsible E3 ubiquitin ligase have common binding requirements in MEIS1 (22). The MEIS family proteins can bind directly or jointly to DNA with a variety of transcription factors. These include various HD-containing proteins, including all HD-containing members of the PAX protein family, as well as OTX2, DLX1, and DLX2, PDX1-specific pancreatic protein, or engrailed in D. melanogaster (23).
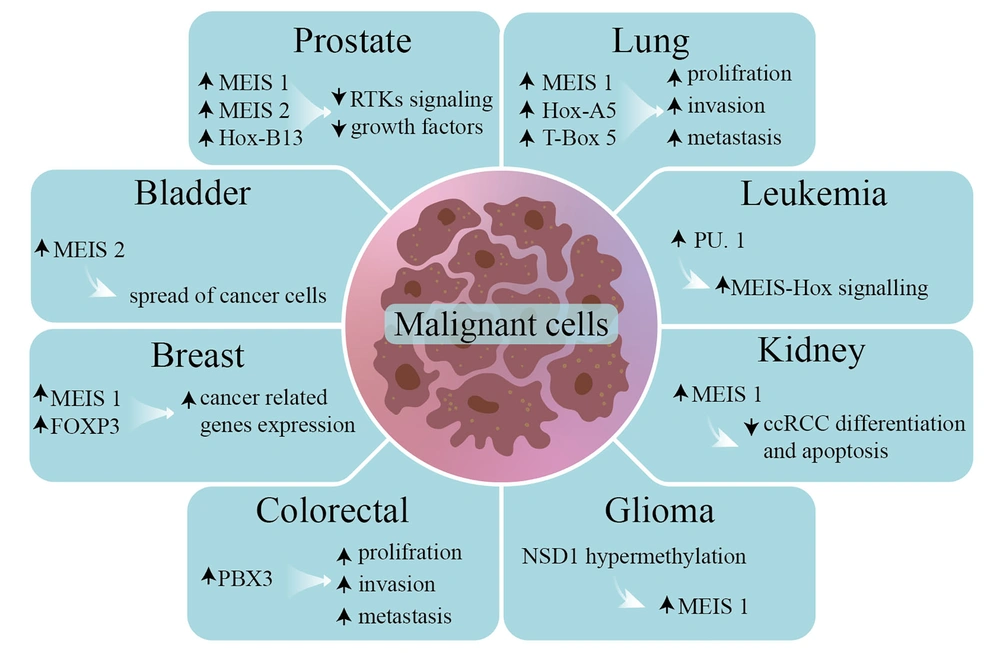
Also, MEIS is associated with other transcription factors such as T-box family member EOMES, basic helix-loop-helix (bHLH) proteins such as MAX, Ets transcription factors such as ELF1, zinc-finger transcription factors such as KLF4 or teashirt (TSH), Transcription factors GATA, NF-Y and nuclear receptors such as Androgen Receptor (AR) in prostate cancer or ecdysone receptor (EcR) receptor in D. melanogaster (Figure 1) (24, 25).
2.1. Bladder
Alternative splicing plays an important role in regulating post-transcriptional genes as well as cancer progression. Polypyrimidine tract binding protein 1 has the function of metastatic bladder cancer and controls alternative splicing, MEIS2, and pyruvate kinase by direct binding to specific introns of these mRNA transcripts and helps to spread bladder cancer (26).
2.2. Breast
In breast cancer, the estrogen receptor causes upregulation of MEIS1 and Forkhead box P3 (FOXP3). Interaction of MEIS1-FOXP3 with a positive feedback mechanism can increase the expression of cancer-related genes in the estrogen receptor pathway (27).
2.3. Colorectal
The PBX3 protein, which forms a heterodimer with MEIS proteins, is overexpressed in colorectal cancers. The PBX3 protein, in particular, by modulating the signaling pathways of mitogen-activated protein kinases (MAPK)/signal-regulated extracellular kinases (ERK), contributes to cell proliferation, invasion, and metastasis in colon cancers (28).
2.4. Glioma
Extinction of protein 1 containing the nuclear receptor SET (NSD1) (nuclear receptor SET domain-containing protein-1) by epigenetic modification leads to Sotos syndrome as well as non-inherited neuroblastoma and glioma. Also, Nsd1 hypermethylation, due to NSD1 not binding to the MEIS1 promoter in neuroblastoma cells, causes upregulation of MEIS1 transcription. High levels of MEIS1 transcript have also been observed in lymphoblastoid cells of patients with Sotos syndrome due to NSD1 silencing (29).
2.5. Kidney
Studies have shown that MEIS1 may act as a tumor suppressor in the progression of clear renal cell carcinoma (ccRCC) because MEIS1 expression is reduced in ccRCC cell lines. In addition, increased MEIS1 expression significantly inhibited the proliferation and apoptosis of ccRCC cells (30).
2.6. Leukemia
As known, MEIS1 is one of the primary factors in the formation of leukemias. Mutations in the MEIS-HOX signaling pathway and its downstream proteins cause MLL. Transcription factor PU. 1 interacts with the MEIS-HOX signaling pathway in leukemia cells, promotes cell cycle progression, and prevents cell death (31).
2.7. Lung
The MEIS1, HOXA5, and T-box 5 proteins are considered markers of pathogenesis in lung cancer adenocarcinoma. Thus, the PBX2 protein, a partner of MEIS1 heterodimer, activates TGF-β, TGF-β-SMAD3, and sonic hedgehog signaling pathways, causing proliferation, metastasis, and cell invasion (32).
2.8. Prostate
Mutation in Hoxb13 poses a vital risk for Prostate Cancer (PC). As known, HOXB13, which enables the activation of the androgen receptor (AR) and Foxa1, forms a heterodimer with MEIS1. The expression levels of butyrylcholinesterase and superfamily member 10 TNF in PC cells are reduced by MEIS1 (33). The MEIS proteins are involved in PC progression by modulating the c-MYC signaling pathway and cell proliferation and are associated with its invasion. Decreased MEIS1 and MEIS2 in vivo may increase tumor growth and increase the expression of the c-Myc and CD142 proto-mogenic genes (34).
2.9. Women
Studies investigating the intricate interactions of MEIS-PBX proteins with HPIP have unveiled their compelling potential to emerge as pivotal biomarkers for the early detection and prognosis of uterine cancer. This research not only deepens our understanding of the disease but also holds promise for the development of targeted diagnostic and therapeutic approaches, offering new avenues for improving the lives of those affected by uterine cancer. High HPIP expression is associated with histological grade, lymph node metastasis, and cancer recurrence. It also reduces overall survival in uterine cancer (35).
2.10. Prostate Cancer
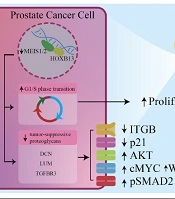
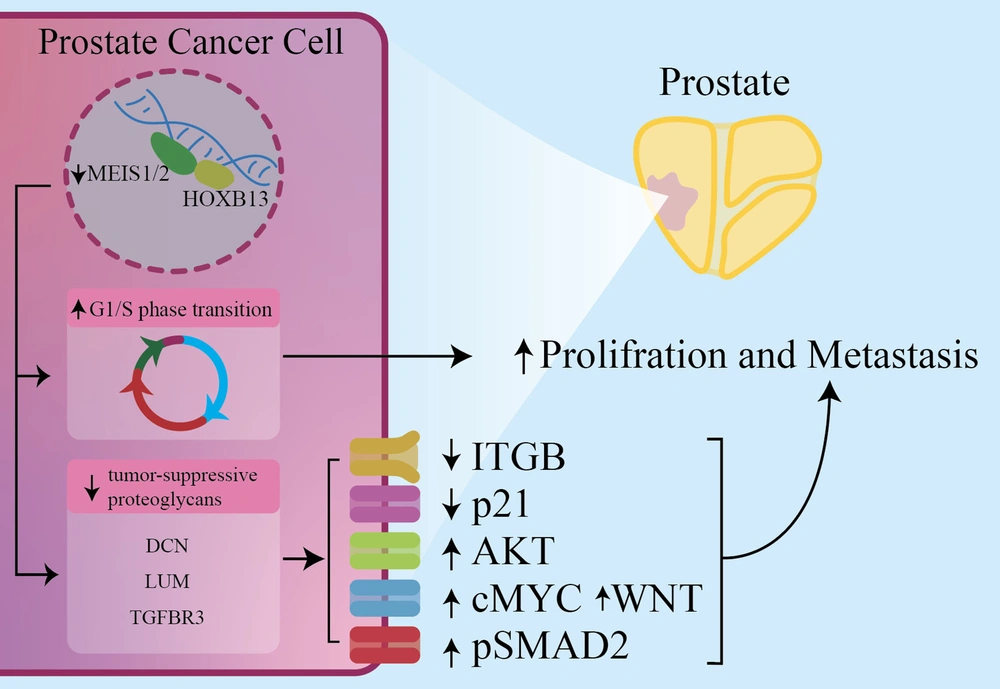
Prostate Cancer (PC) is the most common cancer in men. As known, HOXB13 and Androgen Receptor (AR) expression increases in bone metastatic and primary PCs. Also, HOXB13 is a transcription factor encoded by the HOXB13 gene, found in the genitourinary tract and colon in invertebrates. In normal human prostate cells, HOXB13 plays a developmental role and interacts with MEIS proteins. Alterations in these interactions due to mutations or loss of MEIS expression can lead to PC (36, 37). Prostate epithelial cells have proteoglycans, like decorin (DCN), lumican (LUM), and transforming growth factor-beta receptor III (TGFBR3). Their expression is influenced by the HOXB13-MEIS1 interaction, leading to reduced growth factor and RTK signaling pathways, ultimately decreasing prostate cell proliferation and metastasis. Malignant prostate cells exhibit loss of MEIS1/2 expression, resulting in decreased adhesion, activation of certain signaling pathways, and reduced P21 expression (38). The MEIS1 and MEIS2 expressions inhibit PC proliferation and metastasis by affecting cell cycle and migration. High MEIS levels are associated with non-metastatic PC but not Gleason scores. Reduced MEIS1 expression enhances AR levels, which are crucial in PC development (39). Butyrylcholinesterase (BCHE) plays various roles and decreases in early-stage PC. TNFSF10, a factor involved in apoptosis, is deregulated in prostate cancer. The HOXB13-MEIS1 interaction downregulates TNFSF10 and reduces BCHE levels. The MEIS1 expression is preserved in PC cells, unlike other MEIS paralogues (Figure 2) (40).
In prostate cancer cells (PCCs), downregulation of MEIS1/2 reduces the expression of DCN, LUM, and TGFBR3. This leads to changes in downstream pathways, including a decrease in ITGB and P21 and an increase in AKT, pSMAD2, WNT, and cMYC. These changes lead to an increased proliferation and metastasis potency of PCCs. DCN: Decorin, LUM: Lumican, TGFBR3: Transforming growth factor-beta receptor III (TGFBR3), ITGB: Integrin subunit beta, P21: Cyclin-dependent kinase inhibitor 1, AKT: Protein kinase B, pSMAD2: Phosphorylated Smad2, WNT: Wingless-related integration site.
2.11. Gastrointestinal (GI) Cancers
Gastrointestinal cancers, mainly involving colorectal, esophageal, and gastric cancers, are among the most common cancers in humans and a significant public health burden worldwide (41).
2.12. Colorectal Cancer
Colorectal Cancer (CRC) is a common malignancy with a significant risk of distant metastasis, which contributes to most CRC-related deaths. Unfortunately, there is no reliable indicator to predict the risk of metastasis in patients. Typically, CRC is diagnosed at an advanced stage when metastasis has already occurred, and 25% of CRC patients develop metastases during follow-up.
Research on MEIS proteins in colorectal cancer is limited, but Wang et al. found reduced MEIS2 expression in colorectal cancer tissues compared to normal tissue. They also observed increased methylation at the promoter region of MEIS2 in colon and rectal cancer tissue compared to normal tissue, and there was a negative correlation between MEIS2 expression and the degree of methylation (42). Besides, a cohort study results concluded that BRAF-positive colon tumors, which are mainly located in the proximal colon, showed significant MEIS1 promoter methylation associated with decreased MEIS1 gene expression (43). However, in another study, methylation of the MEIS1 promoter was not detected in 42 colorectal cancer tissue samples (44). In addition, survival analysis showed that MEIS2 downregulation was linked to decreased colorectal cancer patient survival (42).
Studies in Esophageal Squamous Cell Carcinoma (ESCC) have focused on MEIS1 and stemness pathways. The MEIS1 expression in ESCC inversely correlates with SOX2 expression, lymph node involvement, metastasis, and tumor staging, suggesting cancer stemness properties. Also, MEIS1 in ESCC influences factors related to cancer stem-like cells (CSCs). Silencing MEIS1 enhances ESCC differentiation and reduces EMT capabilities (45, 46). In contrast, Zargari et al. demonstrated that the knockdown of MEIS1 in ESCC cells resulted in the under-expression of MEIS1 and other important stemness markers. Based on these findings, MEIS1 may be linked to self-renewal ability in ESCC and suggest a possible oncogenic role for MEIS1 (45). In another study, MEIS1 and Mastermind-like protein 1 (MAML1) mRNA expression levels inversely correlated with tumor size, indicating that larger tumors had lower mRNA expression in these markers. Cases with under-expressed MEIS1 and overexpressed MAML1 were significantly related to tumor depth, with the majority of patients having T3 depth of invasion (47).
2.13. Ovarian Cancer
Ovarian cancer has the highest mortality rate among gynecologic malignancies. On tumor islands and in the stroma, Tumor-infiltrating Lymphocytes (TILs) activate the anti-tumor immune system to control tumor growth (48). Many studies show that TILs are associated with a favorable prognosis for ovarian cancer. The infiltration of CD8+ T lymphocytes, which are a substitute for TILs, is associated with improved survival in ovarian cancer (49). Based on a study by Karapetsas et al., MEIS1/2 is overexpressed in ovarian tumors rich in CD8 + T cells at the early stages (50). Moreover, they found that MEIS1 also contained chemokines, including CCL18/PARC, CCL4/MIP1B (attraction of T-lymphocytes and associated with infiltration of CD + 8 lymphocytes in serous ovarian tumors), CXCL7/NAP2 (attraction of different types of immune cells), CCL5/RANTES, as well as CXCL1/GROα and IL8. This study hypothesizes the cooperation of MEIS1 and SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E member 1 (SMARCE1) in stimulating the promoters of these three chemokines, namely CCL3, CCL4, and IL-8 (51).
The MEIS protein plays a role in proliferation by being expressed in the early proliferative glandular epithelium but not in the rest of the cell cycle. As a result, the overexpression of MEIS1 can enhance tumor growth. According to Crijns et al., the MEIS1/2 proteins are expressed nuclear in the normal ovarian surface epithelium, whereas it is overexpressed both nuclear and cytoplasmic in ovarian carcinomas. Additionally, the presence of the HOXA9–11 protein is exclusive to ovarian cancer cells and is not observed in normal ovarian epithelial cells. The expression of MEIS2 is lower compared to MEIS1, and according to univariate analysis, higher nuclear protein expression of MEIS2 was linked with early-stage and less severe cancers, along with improved overall survival rates (52).
2.14. Leukemia
Hematopoiesis is a highly regulated process by which stem cells differentiate into red blood cells, megakaryocytes, and immune cells from myeloid, lymphoid, or monocyte lineages in the bone marrow or lymphoid tissues. Genetic errors such as chromosomal translocation, chromosomal deletions, point mutations, and epigenetic alterations can stop stem cell maturation at different stages of hematopoiesis and cause uncontrolled proliferation of immature and leukemic immune cells. Leukemia refers to the clonal expansion of leukemic cells in the bone marrow, which classically leads to an increase in the number of cells. Lymphomas are neoplasms of well-differentiated B and T lymphocytes that usually appear as malignant masses in the lymph tissue. Leukemias are generally classified into subgroups defined by cell lineage (lymphocytic or myeloid) and stage of puberty (acute or chronic) (53).
According to GLOBOCAN, leukemia was the 15th most common cancer diagnosed and the 11th leading cause of cancer death worldwide in 2018, accounting for 437,033 cancer cases and 309 006 associated deaths. Globally, the burden of leukemia is higher among men than women. Exposures that are consistently identified as risk factors for leukemia include radiation therapy (therapeutic, occupational, and wartime), chemotherapy, family history, syndromes, genetic disorders, exposure to chemicals (e.g., residential and occupational), and lifestyle-related factors such as smoking (54).
Mutations in the MEIS-HOX signaling pathway and proteins associated with this pathway can cause MLL. Also, the PU.1 transcription factor interacts with the MEIS-HOX signaling pathway in leukemia cells, promotes cell cycle progression, and prevents cell death. In vivo studies have shown that the PU.1 mutation modulates genes. Downstream MEIS-HOX helps expand MLL (31). The MEIS1-PBX and HOX-PBX heterodimer complexes have been shown to occupy the promoter regions of leukemia-related genes. Besides, the MLL1-WD repeat-containing protein 5 (WDR5) protein complex leads to high expression of MEIS1 and HOX due to DNA methylation activity. It was also found that the expression levels of HOX and MEIS1 could be reduced by inhibiting the MLL1-WDR5 protein-protein interaction. Suppose there is an inactive MEIS1 mutation in fetal hepatocytes. In that case, myeloid conversion loses its ability to differentiate and regenerate leukemia stem cells (55, 56).
In Wong et al.'s study using mouse models to highlight the significance of MEIS in MLL leukemia stem cell potential, they found notable variations in the absolute expression levels of MEIS1 across various cells converted to MLL. They observed an increase ranging from a doubling to a remarkable 40-fold rise in expression levels compared to control cells (E2A-HLF). Also, MEIS2 and MEIS3 were expressed but did not continuously increase relative to their levels in E2A-HLF-converted cells, with MEIS1 accounting for 75 to 95% of total MEIS transcripts in MLL-converted cells. Interestingly, MEIS1 transcript levels showed a significant correlation with MLL leukemia delays. Cells converted by MLL fusion proteins that produce short-delayed AML in mouse models showed higher levels of MEIS1 expression. In comparison, those inducing AML with long-term delay showed lower levels of MEIS1 expression. Statistical analysis showed a strong relationship, which indicates that the levels of MEIS1 transcripts decrease rapidly due to the increase in time required to develop leukemia. In contrast, HOXA9 expression levels showed no association with leukemia delays. These results suggest that MEIS1 may play a critical and potentially limiting role in the pathogenesis of MLL leukemia (56).
Studies show that MEIS1 binds to PBX in a DNA-linked transcription complex to modulate MLL transformation. In Wong et al.'s study, they demonstrated that TALE homeodomain proteins play a crucial role in regulating the conversion process of MLL. Specifically, they highlighted MEIS1 as a key regulator influencing the frequency and potential of Leukemia Stem Cells (LSCs). Myeloid ecotropic viral integration site was found to govern various aspects, including self-renewal, differentiation arrest, cycling dynamics, and the overall levels of LSCs within the organism (56). In myeloid cells, MEIS1 is found trimerically with PBX2 and HOXA9. The trimeric complex occupies the region to which DNA PBX2-MEIS1 is attached (57). In addition, there is a regulatory ring associated with MEIS1, along with PU.1, Syk, and miR-146a. The conversion of myeloid precursors to MEIS1 and HOXA9 depends on SYK-induced MEIS1 expression. Also, PBX-MEIS1 heterodimer immortalizes myeloid precursors mediated by HOXA9. In addition, MEIS1-PBX2 interaction increases chemotherapy resistance to leukemia (58). Disruption of MEIS1-HOXA4/9 interaction disrupts epigenetic regulation on chromosomes; therefore, AML formation occurs (59).
As known, MEIS1 and MEIS2 interact with HOX and PBX variants in leukemia. The presence of PBX3 and MEIS1 increases HOXA9-induced leukemia. Also, PBX3 enhances the stability of MEIS1, and the PBX3-MEIS1 heterodimer promotes further transcription of MEIS1. The HOXA9/MEIS1/PBX3 trimmer structure supports HOXA9-induced leukemia (22). In addition, MEIS2 is overexpressed in myeloid leukemia mice, implying that MEIS1 and MEIS2 may have a parallel function in myeloid leukemia (MEIS1 is a very important regulator for the differentiation of hPSCs into cells) (60). A hematopoietic function controls MEIS1 hematopoietic differentiation by targeting T-cell acute lymphocytic leukemia 1 (TAL1) and Friend leukemia integration 1 (FLI1) transcription factors. Also, MEIS2 regulates early hematopoietic differentiation in human embryonic stem cells (16).
The contradictions in the expression of the relationship between the MEIS2 gene and tumors of different body tissues can be attributed to several factors, including the complexity of cancer biology and the context-dependent nature of gene expression. Here are some reasons for these contradictions:
(1) Tumor heterogeneity: Tumors are not uniform entities; they consist of various cell types and subtypes. The role of a specific gene like MEIS2 may vary depending on the tumor type, stage, and genetic mutations present within the tumor. MEIS2's function may be different in different tumor contexts, leading to conflicting findings.
(2) Cell-specific functions: MEIS2 is a transcription factor involved in various cellular processes, including development, differentiation, and regulation of gene expression. Its function can vary in different cell types, and its role in tumorigenesis may differ depending on the cellular context within a specific tumor.
(3) Differing experimental methods and models: Studies investigating the relationship between MEIS2 and cancer may use different experimental techniques and models, which can yield varying results. These discrepancies can arise from differences in sample size, methodology, and the criteria used for data analysis.
(4) Confounding variables: Various factors, such as patient demographics, treatment regimens, and other genetic alterations, can impact the observed relationship between MEIS2 and cancer. These variables are often difficult to control in clinical studies, leading to conflicting findings.
(5) Limited understanding of gene function: Our knowledge of the functions of specific genes like MEIS2 is still evolving. New research may reveal previously unknown roles of MEIS2 in different cancers or provide a better understanding of its context-dependent effects.
(6) Sample size and statistical power: The size of the study cohort can influence the statistical power and the ability to detect significant associations. Small sample sizes may yield inconsistent or inconclusive results.
3. Conclusions
The MEIS agents can absorb a wide range of epigenetic modulators into chromatin during embryonic growth or tissue homeostasis by adult stem cells. Also, MEIS gene activity is fully regulated at multiple levels, including RNA expression, binding, and stability, as well as post-translational protein modification and controlled intracellular availability, all of which are influenced by extracellular factors. The MEIS proteins may act as extracellular signal sensors, quickly converting this information into a transcription result. In various cancers, these proteins and their cofactors are dysregulated. Besides, the MEIS proteins have the potential to be both diagnostic and therapeutic biomarkers for cancer. The estrogen receptor promotes the expression of MEIS1 and Foxp3 in breast cancer. The MEIS1-FOXP3 interaction with a positive feedback mechanism can boost the expression of cancer-related genes in the estrogen receptor pathway. In addition, MEIS1 expression is reduced in clear Renal Cell Carcinoma (ccRCC) cell lines, suggesting that it may act as a tumor suppressor in the progression of the disease. The MEIS proteins and their cofactors are dysregulated in a variety of cancers, and these proteins can be used as diagnostic and therapeutic biomarkers for cancer and other diseases. Hox genes are transcription factors that are distinguished by the presence of a homeodomain, which is a domain of 60 protected amino acids attached to DNA (HD). These genes control cellular fate, growth, tumorigenesis, and stem cell function. Nuclear dynamics, transcriptional regulation, long noncoding RNAs (lncRNAs), RNA processing, miRNAs, and translational events all influence Hox gene expression. As known, MEIS dysregulation may also contribute to chemotherapy resistance in colorectal cancer. Also, MEIS2 and oxaliplatin-based chemotherapy resistance were found to have a significant correlation. In addition, MEIS2 expression was significantly lower in resistant tissues than in sensitive tissues, implying that MEIS2 may be a tumor-suppressive gene in CRC patients. The MEIS proteins promote proliferation by being expressed in proliferative glandular epithelium early in the cell cycle but not later in the cell cycle. As a result, MEIS1 overexpression can promote tumor growth.