1. Background
Stroke, also known as cerebrovascular accident (CVA) or brain attack, is a medical condition where poor blood flow to the brain leads to cell death. Stroke, being the second leading cause of death and the third major cause of disability in adults, imposes significant financial and social burdens on people (1). According to the National and Subnational Burden of Stroke in Iran report from 1990 to 2019, the number of incident cases and deaths is increasing across all Socio-Demographic Index (SDI) quintiles (2). This index combines information on the economy, education, and fertility rate of countries worldwide, representing social and economic development.
The incidence of stroke in developing countries of the Middle East and North Africa is rising, especially at younger ages, due to atherosclerotic or microvascular causes (3-5). Moreover, stroke is the second leading cause of death in Iran at 10.5% (6). The annual incidence of this disease in Iran is 372 per 100,000 individuals, significantly higher than in developed countries (7, 8).
Despite medical advancements, approximately 30% of stroke patients lose their lives, 10% become entirely dependent on others for living, and 60% experience varying degrees of disabilities (9). These adverse events affect the quality of life of patients, their families, and health systems (10). Patients with functional impairment due to stroke require rehabilitation services (11). Rehabilitation at the appropriate time has been demonstrated to improve function, reduce dependency, enhance the quality of life, and increase participation in social activities (12, 13). Considering the increasing demand for health services, especially in the elderly population, managers and policymakers must evaluate methods for managing stroke patients to find the most effective and acceptable approach (14, 15).
Hospital rehabilitation, rehabilitation in stroke units, and home-based rehabilitation are three standard rehabilitation methods in different countries (16). In some developed countries, such as Australia and England, there is a heavy reliance on hospitals for stroke rehabilitation. Strong arguments supporting this method include quick access to precise diagnosis and treatment, as well as nursing care and multidisciplinary rehabilitation, which are more easily provided in hospitals than at home (17). However, the length of hospital stay is the most significant determinant of the direct cost of stroke care (18).
On the other hand, some people advocate for early discharge from the hospital, followed by home rehabilitation. This group highlights several benefits of home rehabilitation, including increased patient satisfaction, reduced risks associated with inpatient care, more focus on rehabilitation outcomes, and lower direct costs such as hospitalization, transportation, and staff expenses (19, 20).
Home-based rehabilitation is more challenging in low- and middle-income countries than in developed countries. Factors such as limited transport options, insufficient finances, long distances to rehabilitation facilities, poor upstream acute stroke care, and low health literacy levels are primary challenges in low-middle-income countries such as Iran (21). This may explain why clinical trial studies have indicated that home-based rehabilitation is less effective than hospital-based rehabilitation (22). Meanwhile, the efficacy of home-based rehabilitation compared to medical centers was demonstrated in 2010 (23). In line with this, European countries found that home-based rehabilitation is accompanied by increased quality-adjusted life years (QALY). Additionally, home-based rehabilitation programs generate substantial cost savings. In the healthcare system alone, these cost savings amounted to €237 million. When considering a broader societal perspective, including costs like social care services, the total cost savings were even greater at €352 million (24).
Specialized stroke units, clinics, or centers are the third method that significantly improves health outcomes (25). Several studies have demonstrated that rehabilitation in stroke units results in improved patient survival (26-28). However, this method is associated with higher costs due to the overhead expenses related to maintaining the rehabilitation center or clinics, infrastructure, utilities, equipment, and staffing costs. Therefore, additional charges will be imposed on the health systems and families if all patients are rehabilitated in stroke units (29). In Iran, some of these stroke units operate in the private sector, and some patients are referred to these centers for rehabilitation services. The Tabasom specialized rehabilitation center in Tehran is currently providing services specifically to stroke patients.
Despite various rehabilitation options for stroke patients, significant challenges persist across different socioeconomic settings. In low- and middle-income countries like Iran, limited resources, workforce constraints, and infrastructure deficiencies hinder comprehensive rehabilitation services. Conversely, the high costs of inpatient and dedicated stroke facility-based care raise sustainability concerns in high-income nations.
While previous studies evaluated specific rehabilitation approaches, a comprehensive economic analysis comparing the costs and outcomes of hospital, home, and stroke unit-based strategies is lacking for the Iranian context. Addressing this knowledge gap through a rigorous cost-effectiveness analysis (CEA) is crucial, given Iran's substantial stroke burden and the need to optimize limited health resources. Furthermore, the findings could catalyze policy shifts and investments to enhance stroke rehabilitation access and efficiency across other resource-constrained, low- and middle-income countries.
2. Objectives
Our results are expected to provide new insights for policymakers in deciding on the best and most cost-effective option for providing rehabilitation services to patients with stroke. The objectives of this study are as follows:
- To evaluate the costs associated with hospital-based, home-based, and stroke unit-based rehabilitation strategies for stroke patients in Iran.
- To calculate and compare the incremental cost-effectiveness ratios (ICERs) of the home-based and stroke unit strategies relative to hospital-based rehabilitation.
- To determine which rehabilitation strategy represents the most cost-effective option based on Iran's willingness-to-pay (WTP) threshold.
3. Methods
3.1. Participants
Participants were selected from patients referred to the Rofaydeh and Tabasom Rehabilitation Centers in Tehran. Their eligibility for the program was based on the following inclusion criteria: (1) stable clinical status, (2) eligibility for rehabilitation services based on the physician's diagnosis, (3) certainty regarding the benefits of rehabilitation services for the patient, (4) no history of previous physical disability before the stroke, (5) stroke as the exclusive cause of disability, and (6) signing informed consent for participation in the study and use of their medical records.
Patients who refused participation, chose to withdraw, or discontinued due to disease progression were excluded. The sampling method was non-probability, with equal sample sizes across groups to reduce bias. Sampling continued until the desired number was reached. Additionally, the groups had the same clinical disability level based on the Modified Rankin Scale (mRS).
3.2. Tools
The mRS was used to evaluate functional status improvement and categorize patients into three health states defined in the Markov model. An mRS of 0 - 2 indicated that the individuals were completely healthy or independent in performing activities of daily living (ADLs). The second group, with an mRS of 3 - 5, consisted of patients who were dependent and required assistance for ADLs. The third group encompassed individuals with an mRS of 6, indicating that they had passed away.
3.3. Procedure
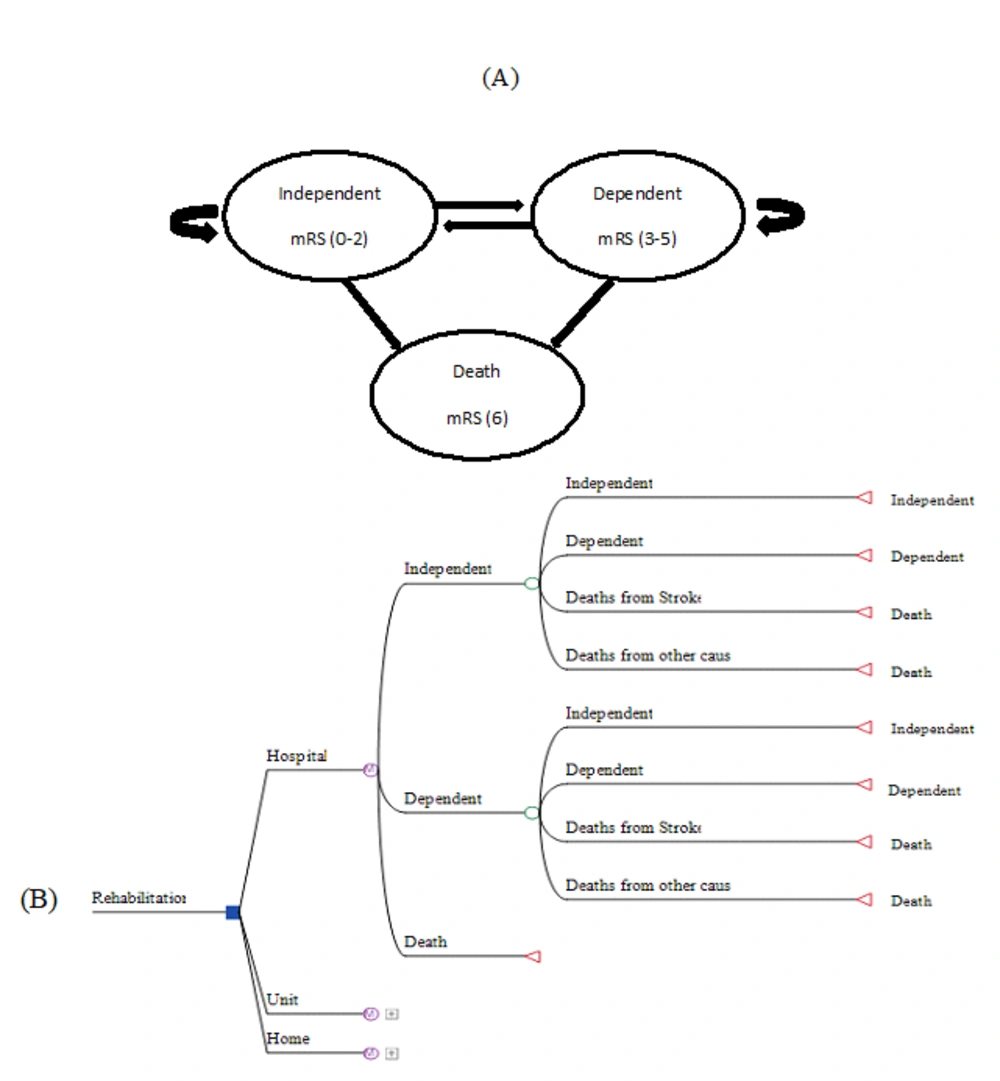
This CEA using the Markov model was conducted from a health system perspective. The Markov model was used due to the similar rehabilitation states in the three rehabilitation strategies (Figure 1A). The model included a decision tree that compared all three strategies (Figure 1B). The Markov model used two types of input information regarding cost and utility. The total costs of the rehabilitation of participants were collected from their medical records, as well as from interviews with patients and relevant experts. The costs included hospitalization, computerized tomography scans, magnetic resonance imaging, echocardiography, medications and supplies, staff time (i.e., physician, nurse, physiotherapist, occupational therapist, speech therapist, nutrition expert, and counselor), and overhead costs (i.e., transport expenditures, medical utilities in the home).
Based on the Markov model, three states were considered in each group of patients. The model consisted of three possible health states for stroke patients: Independent, dependent, and death. These states determine the potential status of people after a stroke, depending on the severity of mRS (30). It should be mentioned that apart from the three primary states of the disease, we incorporated deaths from other causes in the model design using the life table of the World Health Organization for Iran (31). As a result, in each rehabilitation group, namely hospital, home, and stroke unit, there were patients from all three clinical states defined in the Markov model. In addition, the same rehabilitation package was offered to patients in all three groups. The rehabilitation package across settings consisted of physical therapy focused on mobility and self-care tasks, occupational therapy for ADLs, speech therapy for communication and swallowing, counseling, and medical and nutritional services if needed.
Life expectancy in Iran is 75 years, and the mean age of the participants in the present study was 55 years. Consequently, the time horizon was considered to be 20 years. According to previous studies, the length of the Markov model cycle was set to three months (32).
The WTP threshold refers to the maximum amount a country is willing to pay per unit of health outcome (e.g., per quality-adjusted life year or QALY) to consider a healthcare intervention or technology cost-effective. The country's threshold, or willingness to pay, is three times the country's gross domestic product (GDP) per capita, equal to $18,341.80.
As our study is a secondary study [health technology assessment (HTA), which is one of the Ministry of Health's priority research areas] and we do not have proprietary stroke QALY/utility data for Iran, we reviewed previous studies for transition probabilities and utility values. The Markov model's transition probabilities between health states were derived from published data on outcomes in stroke populations undergoing rehabilitation. We chose these parameters (transition probabilities and utilities) from the studies by Allen et al. and Amiri et al., which were compatible with our research (33, 34). They reported rates of functional status changes over three months in stroke survivors (33, 34). These probabilities were adapted to our 3-month model cycles.
The primary advantages of Markov analysis are its simplicity and out-of-sample forecasting accuracy (35). Future health is often considered less valuable than immediate health (35). This is handled in some models by discounting future utility by a constant rate. We evaluated discount rates of 0.03 and 0.06 for utilities and costs, respectively (36). Therefore, the final and incremental costs with a discount rate of 0.06 and the final and incremental utility with a discount rate of 0.03 for a 20-year time horizon in 80 cycles were calculated and entered into the model. All costs were calculated based on the value of the US dollar in 2019.
3.4. Ethical Considerations
This study utilized de-identified patient data from medical records. Appropriate ethical approval was obtained from the hospital ethics committee. Informed consent was collected from all participants.
3.5. Statistical Analysis
3.5.1. Cost-Effectiveness Analysis
The ICER and the average cost-effectiveness ratio (ACER) were calculated using TreeAge Pro 2013 software to determine which rehabilitation method should be accepted. The CEA results are presented per 1000 patients to provide context on the scale of the analysis.
We calculated the ICER between the options using the difference in costs in 2019 International Dollars divided by the difference in effectiveness in quality-adjusted life years gained.
ICER Home care = (Cost Home care - Cost Hospital)/ (Effect Home care - Effect Hospital)
Although the ICER is more relevant to health economics and policy decisions, the ACER has several advantages that should be noted: (1) it characterizes the clinical and economic properties of treatment independent of its comparators, making it straightforward to apply to one group or more than two groups; (2) researchers and policymakers may want to see the ACER (e.g., for short- vs. long-term costs) even when the ICER is used for decision-making; (3) it is less vulnerable to numerical instability compared to the ICER (37).
Therefore, we also calculated the ACER of each alternative using the ratio of the cost to benefit of each intervention.
ACER Home care= Cost Home care/Effect Home care
3.5.2. Sensitivity Analysis
Regardless of the accuracy used in the CEA, there is usually uncertainty associated with input parameter values. Consequently, sensitivity analysis was used to determine the effect of these uncertain parameters on the study results. One-way and two-way sensitivity analyses, as well as tornado diagrams, were conducted in addition to probabilistic sensitivity analysis (PSA) using a 20% variation. The acceptability curve, a form of PSA, was also conducted. It illustrates the uncertainty around the ICER by showing the likelihood that the ICER will fall below different cost-effectiveness thresholds. The distribution of variables in the model for PSA analysis was determined using EasyFit 5.5 professional software.
4. Results
4.1. Demographic Information
Table 1 shows the demographic information of 210 patients participating in this study.
| Demographic Characteristics | Hospital (n = 70) | Stroke Unit (n = 70) | Home-Based (n = 70) | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Women | 34 | 40 | 28 | 48 | 39 | 55 |
| Men | 36 | 52 | 42 | 60 | 31 | 45 |
| Functional status | ||||||
| Independent | 25 | 36 | 45 | 64 | 22 | 31 |
| Dependent | 41 | 59 | 23 | 33 | 47 | 67 |
| Death | 4 | 6 | 2 | 3 | 1 | 1 |
| Age | 51.1 ± 2.8 | 55.2 ± 1.3 | 60.1 ± 24 | |||
Demographic Characteristics and Functional Status of Patients Receiving Home-Based, Stroke Unit, and Hospital Rehabilitation Services a
4.2. Direct Costs
The costs for each of the three methods were as follows:
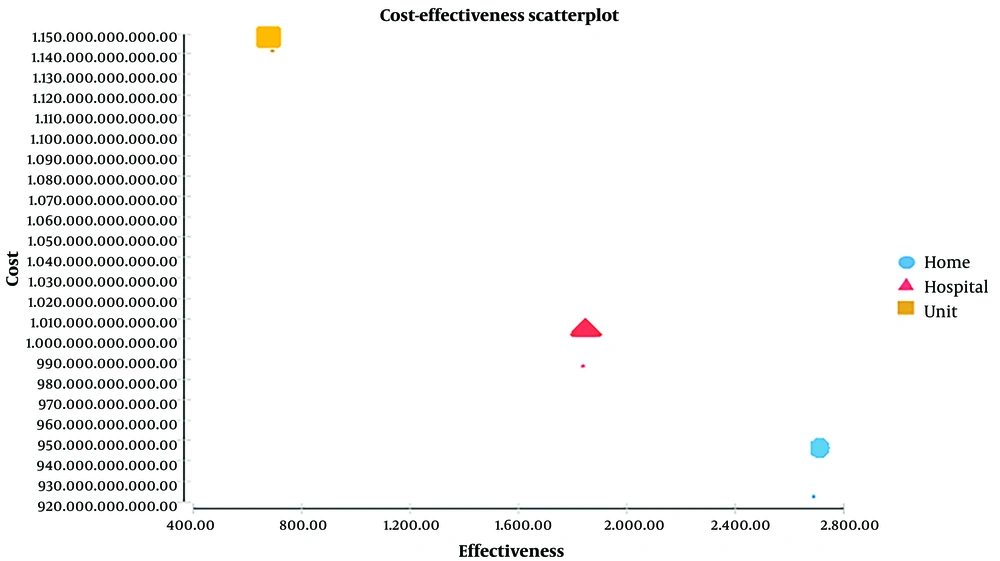
The total direct cost (expenses associated with production and sales) per patient for the hospital, stroke unit, and home strategies were $2955 ± 48.18, $3485 ± 51.63, and $2306 ± 35.018, respectively. The cost of each of the three health states was calculated in each group to evaluate cost-effectiveness using the Markov model.
4.3. Cost-Effectiveness Analysis
Table 2 shows the results of the CEA of the rehabilitation of patients with stroke delivered in hospitals, homes, and stroke units.
| Strategy | Effectiveness Quality-Adjusted Life Years (QALYs) | Cost ($) | ACER | ICER |
|---|---|---|---|---|
| Hospital a | 17.99 | 205527 | 11424 | |
| Stroke unit | 7.03 | 233114 | 33159 | 2517.06 |
| Home | 26.03 | 188277 | 7233 | 2145.52 |
Cost-Effectiveness Analysis Results Comparing Home-Based, Stroke Unit, and Hospital-Based Rehabilitation Strategies for Stroke Patients in Iran, per 1000 Patients Over a 20-year Time Horizon
According to Table 2 and the mentioned WTP threshold, the ACER of rehabilitation at hospitals, stroke units, and home groups are $11,424, $33,159, and $7,233 per QALY, respectively.
The ICER of stroke unit rehabilitation relative to hospital rehabilitation was $2,517 per QALY, which indicates that over a 20-year time horizon, 10.96 QALYs have been obtained, and costs have decreased by $27,587 in the hospital group per 1,000 patients.
The ICER of home rehabilitation relative to hospital rehabilitation was $2,145 per QALY, which shows that over a 20-year time horizon, 19 QALYs have been obtained, and costs have diminished by $17,250 in the home-based method compared to hospital-based methods per 1,000 patients.
4.4. Sensitivity Analysis
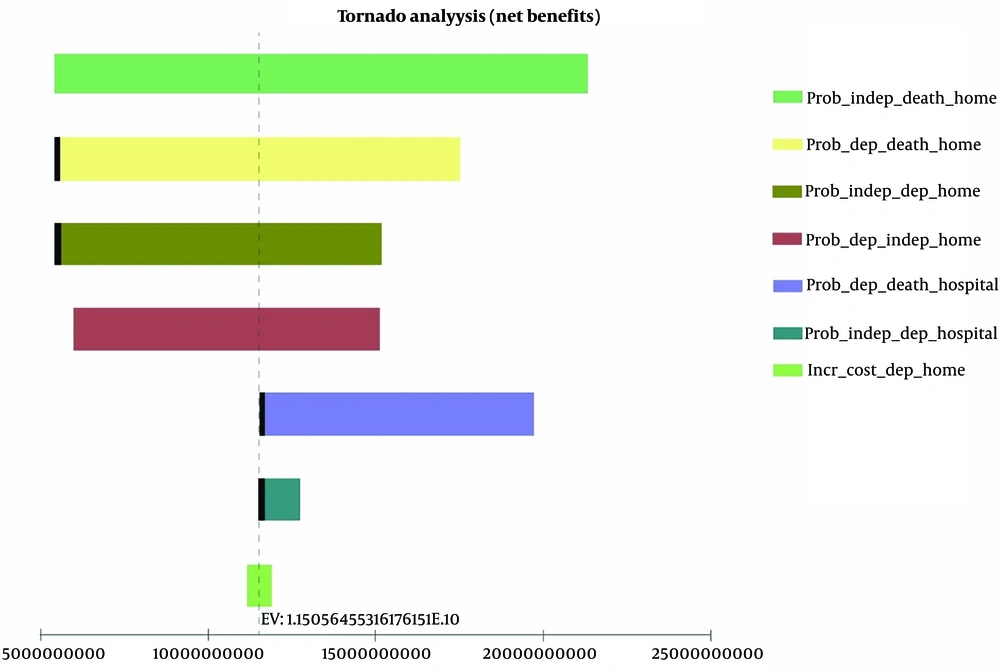
The sensitivity analysis was conducted for all uncertain parameters of the model, including utility, cost, transition probability, and initial distribution, based on the variation range presented in Table 3. According to the tornado diagram, the model had the highest sensitivity to the transition probabilities of different home states, the transition probabilities of other hospital states, and the incremental cost of dependent states at home (Figure 2). One-way and two-way sensitivity analyses showed the most significant effect on the ICER. Changes in these parameters did not influence the results of the investigation.
| Parameter | Base Case | Variation Rrange | Distribution | References Number | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| Transition probabilities | |||||
| Home | |||||
| Dependent to death | 0.05 | 0.36 | 0.00 | 23 | |
| Dependent to independent | 0.12 | 0.61 | 0.00 | 23 | |
| Independent to death | 0.017 | 0.27 | 0.00 | 23 | |
| Independent to dependent | 0.031 | 0.44 | 0.00 | 23 | |
| Hospital | |||||
| Dependent to death | 0.05 | 0.36 | 0.00 | 23 | |
| Dependent to independent | 0.06 | 0.41 | 0.00 | 23 | |
| Independent to death | 0.017 | 0.27 | 0.00 | 23 | |
| Independent to dependent | 0.10 | 0.90 | 0.00 | 23 | |
| Unit | |||||
| Dependent to dependent | 0.7407 | 0.81 | 0.65 | 22 | |
| Dependent to independent | 0.1111 | 0.18 | 0.03 | 22 | |
| Independent to dependent | 0.0938 | 0.11 | 0.07 | 22 | |
| Independent to independent | 0.875 | 0.89 | 0.77 | 22 | |
| Cost ($) | |||||
| Home | |||||
| Death | 1970 | 2363 | 1575 | ||
| Dependent | 4678 | 5613 | 3742 | Gamma | |
| Independent | 2693 | 3231 | 2154 | Normal | |
| Hospital | |||||
| Death | 2295 | 2754 | 1836 | ||
| Dependent | 3868 | 4641 | 3094 | Log-normal | |
| Independent | 2702 | 3242 | 2161 | Uniform | |
| Unit | |||||
| Death | 2943 | 3531 | 2354 | ||
| Dependent | 4500 | 5399 | 3599 | Normal | |
| Independent | 3113 | 3615 | 2410 | Log-normal | |
| Utility | |||||
| Home | |||||
| Dependent | 0.56 | 0.94 | 0.13 | Beta | 23 |
| Independent | 0.79 | 1.00 | 0.52 | Beta | 23 |
| Hospital | |||||
| Dependent | 0.65 | 1.00 | 0.22 | Beta | 23 |
| Independent | 0.79 | 1.00 | 0.51 | Beta | 23 |
| Unit | |||||
| Dependent | 0.38 | 0.58 | 0.18 | Uniform | 22 |
| Independent | 0.74 | 0.94 | 0.54 | Uniform | 22 |
Parameters for Sensitivity Analysis per Patient in Markov Model
Exact transition probability values and sources with 20% sensitivity are shown in Table 3. This allowed us to evaluate the impact of fluctuating transition rates on model outcomes. Probabilistic sensitivity analysis was conducted on uncertain variables with a specific distribution for 10,000 cohorts using Monte Carlo simulation (Table 3 and Figure 3)
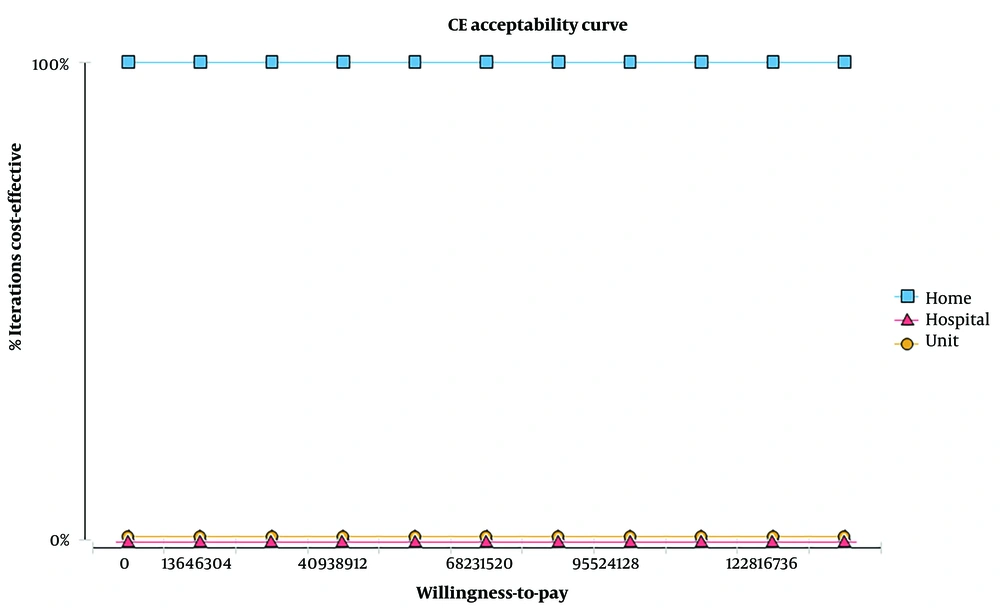
Furthermore, the findings of the acceptability curve (Figure 4) indicate that home-based rehabilitation has the highest willingness to pay for patients and is consistently regarded as a cost-effective strategy across various thresholds.
5. Discussion
Our findings indicate that from a healthcare system perspective, home-based rehabilitation is the most cost-effective option compared to the other two strategies. Providing home-based rehabilitation is both less costly and more effective. These findings align with the results presented by Patel et al., which showed that home care was the least expensive option compared to stroke units and general wards. Both incremental CEA and cost-utility analyses supported the cost-effectiveness advantage of home-based rehabilitation (38). Moreover, Allen et al. evaluated the impact of a home-based stroke rehabilitation program and indicated that home-based rehabilitation is more cost-effective than conventional services at the hospital (33).
More broadly, this study provides evidence to support greater investment in home-based models of stroke rehabilitation across low- and middle-income countries facing high stroke burdens and health system constraints. Policy initiatives to enable safe and quality home-based care, rather than building specialized facilities, can allow more patients to access needed rehabilitation services.
It seems that hospital-based rehabilitation services impose additional costs and are associated with lower efficacy. According to the available evidence, long-term hospitalization of stroke patients leads to the involvement of other people, such as family members or friends, in addition to the patient. These conditions have variable effects on a family's economic situation and daily performance (39, 40). Furthermore, longer hospitalization is accompanied by an increased risk of infection development. This issue is exacerbated in Iran due to higher antibiotic consumption and subsequent resistance to infection treatment (40). Poor sanitation, limited disposables, contaminated blood products, and the inappropriate use of pressure sterilizers and antibiotics further raise costs for patients and hospitals (41, 42).
On the other hand, from the perspective of the health system, direct hospital costs are high due to medical services. These costs include medical tests, speech therapy, physiotherapy, neurology, medical specialists, and their assistants (43-45). Moreover, direct non-medical costs, including transportation, lodging, and food for these patients in the hospital, are also prohibitively high (46, 47).
The differences in QALYs between strategies arise primarily from more patients starting and remaining independent over time with home rehabilitation compared to hospital or stroke unit care. Home rehabilitation results in a 26.03 QALY outcome compared to only 17.99 QALYs for hospital care over 20 years. This suggests that home rehabilitation is better able to help dependent individuals regain independence for daily living.
While Iran shares stroke risk factors and rehabilitation options with other upper-middle-income nations, our findings suggesting the cost-effectiveness of home-based rehabilitation likely have the greatest applicability to low- and middle-income countries facing healthcare financing constraints, especially in the Middle East and North Africa region. However, direct generalizability may be limited by setting-specific variations in reimbursement policies, workforce availability, and caregiver support capacities (48). The discussion explores these nuances to contextualize the relevance across diverse resource-constrained environments. The cost-effectiveness findings favoring home-based rehabilitation are most relevant for countries reliant on hospital-based stroke rehab with similar health expenditures as Iran, such as nations in Latin America and Central/Eastern Europe (49, 50). Considering each country's stroke burden and health system context remains crucial for assessing generalizability.
For Iran's health system context, home-based rehabilitation is the preferred strategy across most WTP thresholds, with hospital-based care only potentially cost-effective above $25,000 per QALY. However, implementing home services requires addressing clinical challenges like monitoring adherence, responding to complications, and assessing home safety (51). Implementation hurdles include obtaining patient buy-in, building workforce and infrastructure, and developing care coordination processes (52). To facilitate this transition, strategies include financial incentives, pilot projects, clear quality standards, workforce training expansions, care coordination investments, caregiver support services, and tele-rehabilitation policies enabling remote access (53).
Addressing these challenges will require ongoing engagement with clinical, community, industry, and government stakeholders. Therefore, it can be said that home rehabilitation reduces the hospitalization rate and length of hospital stay, in addition to avoiding hospitalization costs. Furthermore, home-based rehabilitation can improve the performance of patients, enhance their quality of life, and promote outcomes based on the results of the present study and other available evidence (54, 55).
5.1. Limitations
The lack of data on the utility and quality of life of patients with stroke in Iran is a limitation of the current investigation. As a result, we had to use utility values from other studies. Using specific utility data for patients with stroke in Iran could help to obtain a more realistic analysis of this issue. We attempted to reduce the effect of these limitations and elevate the validity of the results by conducting a thorough PSA and selecting studies with settings similar to our research.
5.2. Conclusions
The findings of this study demonstrated that home-based rehabilitation is a more cost-effective strategy for stroke patients compared to inpatient rehabilitation settings. The lower costs and higher long-term quality of life provide a strong rationale for healthcare policymakers and public health officials to prioritize the development of home-based rehabilitation programs.