1. Background
Anemia arising from iron deficiency stands as one of the most pervasive health disorders, significantly contributing to global disability, particularly among women, where it ranks as the primary cause. The World Health Organization (WHO) estimates that two billion individuals worldwide suffer from anemia, with iron deficiency accounting for half of these cases (1-4). Approximately 40% of pregnant women and 30% of non-pregnant women are affected by iron deficiency, underscoring the gravity of the issue (1, 5, 6). Iron, a fundamental trace element, plays a pivotal role in vital cellular processes, including energy acquisition and oxygen transport. Recognizing the magnitude of this health concern, it becomes imperative to address and act upon the enduring prevalence of anemia across diverse regions globally (6-8).
In iron deficiency anemia, serum iron levels typically decrease due to inadequate dietary intake, poor absorption, or chronic blood loss. This leads to a significant drop in serum ferritin levels, indicating depleted iron reserves (9, 10). However, ferritin levels can also be affected by inflammation, as it is an acute-phase reactant (11, 12). Total iron-binding capacity (TIBC) measures the capacity of serum proteins to bind iron. In iron deficiency anemia, TIBC often increases as the body attempts to capture more iron from dietary sources or internal stores (13, 14).
The concentration of iron in saliva is generally lower than in serum, but it can still reflect the body's iron levels to some extent (15). Changes in salivary iron levels during anemia are not as well-studied as those in serum and are less pronounced. Salivary ferritin levels are usually very low and are not as reliable as a direct marker of body iron stores compared to serum ferritin. However, recent studies have investigated its potential use in non-invasive diagnostics (4, 16-20). Salivary TIBC is an area of emerging research (21-24). Some studies suggest that salivary TIBC might be correlated with the body's iron-binding capacity (21-23), but the mechanisms and reliability are not yet fully understood.
The diagnosis of anemia can be challenging due to its nonspecific symptoms (4, 16). Currently, diagnosis requires multiple assessments of iron and ferritin levels, including a comprehensive blood cell count (CBC), peripheral smear, iron indices, and bone marrow iron microscopy. Unfortunately, this method necessitates the invasive extraction of venous blood, which entails practical and psychological complexities and poses a substantial risk of contamination. These impediments may prevent healthcare professionals from achieving early diagnoses and implementing timely treatments. In response to these challenges, recent initiatives have sought to replace blood-centric investigations with alternative biological samples that do not have such drawbacks (16-20, 25-27).
In previous studies, Ishikawa observed that in healthy adults in Japan, urine ferritin levels were 5% of the corresponding serum ferritin levels, showing a correlation coefficient of 0.79 (25, 26). Skalny et al. (27) analyzed the potential of laser-induced breakdown spectroscopy (LIBS) for trace minerals in biosamples in a recently published review article. They found that LIBS can be used to trace iron and other minerals in teeth, bones, nails, and hair samples (27). In 2024, Prakobdi et al. successfully developed a novel, non-invasive saliva-based diagnostic approach for iron deficiency anemia screening using a nitrocellulose lateral flow system and ferroin reaction (28).
Saliva has been suggested as a potential diagnostic tool for detecting iron deficiency in some studies (4, 16-20). Saliva, a biofluid originating from the vasculature that nourishes the salivary glands, reflects changes in serum composition during disease processes. Evidence suggests that certain drugs, such as deferiprone, exhibit comparable pharmacokinetic profiles in both serum and saliva (29). This revelation underscores the potential of saliva as an alternative diagnostic tool for monitoring various diseases, offering advantages such as ease of collection, cost-effectiveness, and procedural simplicity (3, 18-20, 30). While there are numerous studies on the utility of saliva as a diagnostic measure for anemia in children (18, 30-32), there is a conspicuous absence of studies for adults. This raises questions about the possibility of using this biosample as a diagnostic tool in adults and its procedure.
2. Objectives
This study aimed to ascertain the viability of saliva as a predictive marker for monitoring iron levels in iron deficiency anemia. This involved a comprehensive examination, comparison, and correlation of iron, ferritin, and TIBC levels in the serum and saliva of both iron-deficient anemic and healthy adult individuals.
3. Methods
3.1. Ethical Approval
This study resulted from two projects both of which were registered and approved by the ethical committee under the ethical codes of IR.IAU.DENTAL.REC.1398.024 and IR.IAU.DENTAL.REC.1398.025.
3.2. Sample Size
In this comparative analysis study, 40 women were analyzed. The sample size was calculated as 20 for each group based on Kodati’s study (18), using SPSS software with a 95% confidence interval and 80% test power.
3.3. Study Population
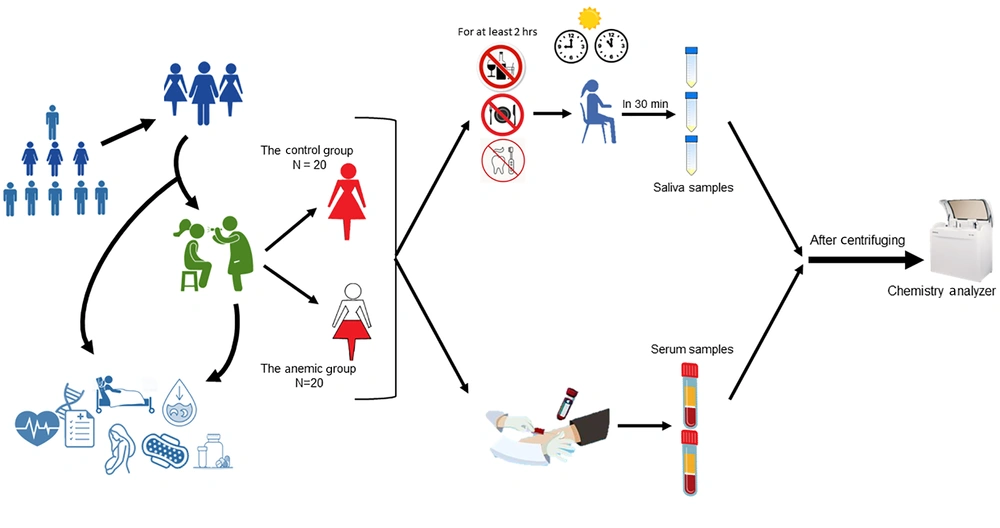
Forty women aged 20 to 40 were selected using a purposeful sampling method and divided into two groups: (1) anemic and (2) healthy (control). Since periodontal problems can alter saliva composition and affect serum ferritin levels, all subjects were examined before sampling using a periodontal probe and evaluating clinical attachment level (CAL). Only those with the same periodontal status were selected.
3.4. Eligibility Criteria
The anemic group included 20 women with iron-deficiency anemia recently diagnosed by a hematologist using CBC, serum iron test, and TIBC. All the subjects had serum ferritin levels of less than 25 ng/mL. They had no history of other systemic disorders and were not taking any systemic drug therapy. The control group consisted of 20 women with the same periodontal status as the anemic subjects, no history of systemic diseases, and normal blood tests. Subjects with hemoglobinopathies, infections, sickle cell anemia, thalassemia, transfusions, or those who were pregnant, on a special diet, in their menstruation or menopause period, taking any medications, or showing any abnormalities in blood components were excluded from the study (31-33).
3.5. Study Procedures
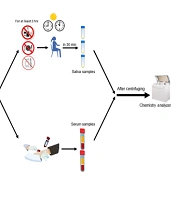
After explaining the procedure to the participants and obtaining informed consent, non-stimulated saliva samples were collected using the spitting method between 9 - 11 am (18). Subjects had not eaten, drank, or brushed their teeth for at least two hours before the saliva collection. Following the standard method of saliva sampling, subjects sat, and three milliliters (mL) of their saliva were collected in Falcon tubes (Maxwell Inc.; Malaysia) over 30 minutes. These samples were transferred to the laboratory within two hours. They were then centrifuged at 4000 rpm for 10 minutes and stored at -8°C (34). The saliva samples' iron, ferritin, and TIBC levels were evaluated using a chemistry analyzer (Mindray BS-380; China) (Figure 1).
3.6. Data Analysis
All data were transferred to SPSS 24 software. The normality distribution of the data was analyzed using the Kolmogorov-Smirnov test. A T-test was used to compare iron, ferritin, and TIBC levels in the serum and saliva of the two groups. Pearson's correlation test was used to investigate the relationship between iron, ferritin, and TIBC values in serum and saliva in both the control and anemic groups. The level of significance was set at 0.05.
4. Results
In the present study, an analysis of the saliva and serum components of 40 female subjects was conducted, focusing on iron, ferritin, and TIBC levels. The results are summarized in Table 1, which presents the minimum, maximum, and mean ± SD values for the two groups under investigation. The chi-square test revealed no statistically significant difference in the ages of the subjects between the two groups (P-value = 0.33) (Table 1).
| Groups | Sample Size | Minimum - Maximum | Mean ± SD | P-Value |
|---|---|---|---|---|
| Age | 0.33a | |||
| Anemic | 20 | 20 - 40 | 30.40 ± 6.36 | |
| Control | 20 | 20 - 38 | 27.75 ± 5.27 |
a Chi-square test.
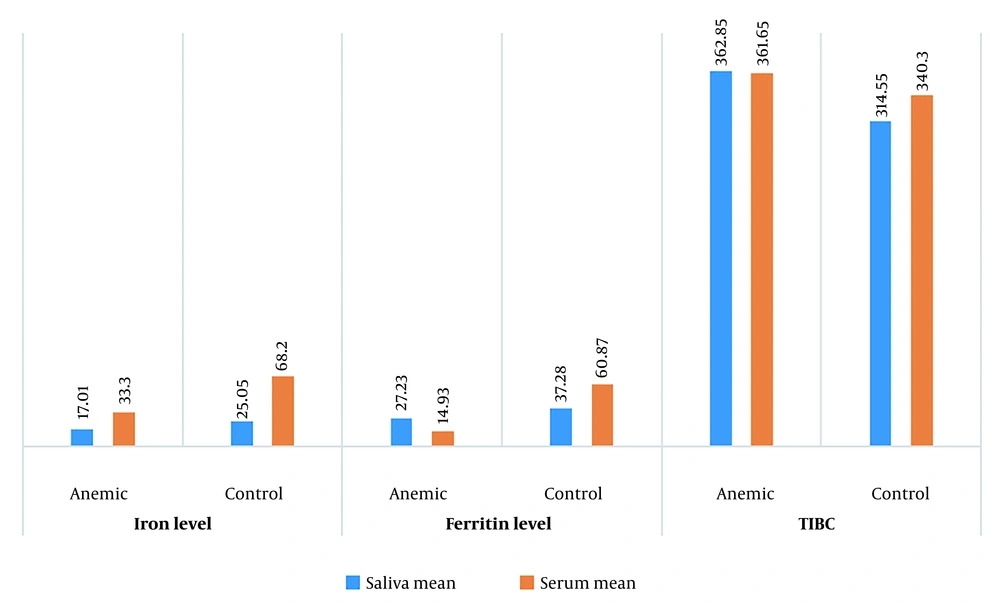
Examining the iron and ferritin levels in saliva, no significant difference was observed between the anemic and healthy groups (P-value = 0.07 and 0.26, respectively). However, an independent T-test demonstrated a substantial difference in TIBC levels in saliva between the two groups (P-value = 0.001) (Table 2 and Figure 2).
| Saliva Substances | Sample Size | Mean ± SD | P-Value |
|---|---|---|---|
| Iron level | 0.07 | ||
| Anemic | 20 | 17.01 ± 11.35 | |
| Control | 20 | 25.05 ± 15.71 | |
| Ferritin level | 0.26 | ||
| Anemic | 20 | 27.23 ± 29.61 | |
| Control | 20 | 37.28 ± 26.48 | |
| TIBC | 0.001 a | ||
| Anemic | 20 | 362.85 ± 45.20 | |
| Control | 20 | 314.55 ± 33.88 |
a Independent t-test.
Evaluating the serum components in both anemic and healthy subjects revealed a statistically significant difference between the groups in terms of iron and ferritin levels (P-value = 0.0001 for both). Interestingly, the comparison of TIBC levels in serum showed no statistically significant difference between the two groups (P-value = 0.118) (Table 3 and Figure 2).
a Independent t-test.
These findings underscore the importance of examining both saliva and serum components when assessing iron status. While certain parameters, such as iron and ferritin levels, exhibit noteworthy distinctions in serum between anemic and healthy individuals, the TIBC levels in saliva emerge as a potentially distinctive marker. This dual-modal analysis provides a comprehensive understanding of the nuanced variations in iron-related parameters, contributing valuable insights to the diagnostic landscape of iron deficiency anemia.
5. Discussion
Many women in their reproductive age face the difficulties of iron deficiency and iron deficiency anemia. This condition can lead to numerous negative health outcomes that affect both their physical and emotional well-being (35). Thus, accurate diagnosis and appropriate treatment are crucial to prevent complications (36).
The primary method for diagnosing anemia is evaluating serum ferritin and iron levels, considered the gold standard test (19). Nevertheless, this approach has certain drawbacks. It requires the collection of blood samples through invasive venous blood extraction, which involves inserting a needle into a vein, causing discomfort and posing a risk of infection or contamination (16-20).
Saliva is widely recognized as a biological diagnostic fluid, appreciated for its uncomplicated, non-invasive, and user-friendly sample collection method, along with its relatively affordable nature (37). Previous studies have shown a correlation between serum and saliva iron levels in both iron-deficient and iron-overloaded groups. Mishra et al. (38) and Canatan and Akdeniz (32) found a strong correlation between serum and saliva iron levels. Jazaeri et al. (21) indicated that saliva can be used as a diagnostic tool for iron deficiency anemia since it is linked to serum iron levels.
Consistent with these previous studies, our research highlighted that the mean levels of iron and ferritin in the saliva of the anemic group were lower than those in the control group, which aligns with the results obtained from the serum analysis (Tables 2 and 3). However, unlike the studies conducted previously, our findings showed that this disparity was not statistically significant. The results also showed a statistically significant difference between the two groups regarding saliva TIBC levels.
Despite the lack of statistical significance in the differences of saliva iron and ferritin levels between anemic and healthy groups, the findings have important implications. Firstly, the study underscores the potential of saliva as a non-invasive diagnostic tool, which could be particularly useful in large-scale screenings or in settings where blood collection is impractical. However, there are limitations in interpreting the results. The study found that saliva iron and ferritin levels may not be reliable in differentiating between individuals with iron deficiency anemia and those with normal iron status if TIBC levels are not measured simultaneously.
Saliva-based diagnostic tools that only measure these markers without considering TIBC may lack sensitivity and lead to false-negative results. Individuals with iron deficiency anemia may have normal or near-normal saliva iron and ferritin levels, resulting in a failure to identify the condition using iron and ferritin markers alone. Therefore, integrating multiple diagnostic approaches and biomarkers may provide a more comprehensive assessment of iron status and improve the accuracy of anemia diagnosis. Biomarker levels in saliva are often significantly lower than in blood, so achieving accurate and stable saliva testing requires consideration of factors such as interferences, matrix effects, viscosity, salivary flow rate, and food consumption (39).
Secondly, the results highlight the need for additional research to better understand the relationship between salivary and serum iron markers. Future studies with larger sample sizes or different demographic groups may provide more definitive insights. Additionally, exploring other salivary biomarkers or refining analytical methods could enhance the sensitivity and specificity of saliva-based diagnostics for iron deficiency anemia. There is a limited amount of research on this subject, particularly studies involving adults. Most of the studies conducted in this area involve children (8, 20, 31, 38). Therefore, more extensive research is needed to evaluate the effectiveness of saliva as a diagnostic tool in adults.
We selected all participants based on the same periodontal status. The progression of periodontal disease, as defined by pocket depth, gingival bleeding, and suppuration, is associated with the level of salivary biomarkers (40, 41). Bleeding gums caused by periodontal issues can affect the measurement of iron levels in saliva samples. Blood contamination can lead to inaccurate results, compromising the reliability of the study.
Additionally, periodontitis is an inflammatory disease believed to be connected with the level of serum ferritin. The expression of ferritin is controlled by various factors, including proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6, which play a crucial role in periodontitis (42).
While this study has its strengths, it also has a few limitations. We tried to eliminate the impact of any confounding factors by matching the examined groups and applying various exclusion criteria. While this approach can help control for confounding factors and enhance the internal validity of the study, it may also limit the external validity or generalizability of the findings to broader populations. Future research should aim to include more diverse patient populations to better understand the utility of saliva analysis for diagnosing and monitoring iron deficiency anemia in real-world clinical practice.
5.1. Conclusions
The results underscore the necessity of combining both serum and saliva analyses to obtain a holistic perspective on iron-related parameters. While saliva analysis cannot entirely replace serum analysis, it offers a convenient approach that can serve as an initial step or be integrated into extensive anemia screening programs, considering its limitations. This methodology has multiple advantages, such as ease of collection, cost-effectiveness, and procedural simplicity, making it especially valuable in large-scale initiatives.