1. Background
Coronavirus disease 2019 (COVID-19), one of the biggest health crises in human history, has aroused a passionate interest among researchers and clinicians to elucidate the short and long-term effects of the disease on various human body organs (1). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection induces a long-lasting inflammation that persists for several months after the initial viral infection, a condition termed long COVID syndrome (2). Long-lasting inflammation is characteristic of long COVID-19-induced manifestations, named post-COVID sequelae, which may lead to serious complications, including infertility and ovarian suppression, requiring careful examination to reveal the extent of its effects on female reproduction (3, 4).
A systematic review of different populations investigating the effects of COVID-19 infection on women’s fertility during the post-COVID recovery period emphasized the need for an extensive evaluation of the long-term effects of the disease on female fertility across various populations (5). The female reproductive system, through the expression of ACE2 (angiotensin-converting enzyme 2), the main receptor for SARS-CoV-2, is susceptible to virus invasion. The virus potentially interferes with basic female reproductive aspects, including folliculogenesis, steroidogenesis, endometrial shedding, and regeneration (6).
The surface spike of SARS-CoV-2 binds to cellular ACE2, which acts as both an enzyme and surface receptor, allowing the virus to enter host cells through the cell surface protein TMPRSS2. This process changes the structure of the host cells and enables local viral replication (7). ACE2 is widely expressed in the ovary, uterus, vagina, and placenta. Co-expression of ACE2 and TMPRSS2 in oocytes and ovarian granulosa cells shows the susceptibility of these cells to SARS-CoV-2 (6, 7). Infection leads to reduced expression of ACE2, resulting in a rise in circulating Ang II and consequently elevating reactive oxygen species and oxidative stress, which can disturb ovarian physiology by triggering inflammatory reactions (8).
Severe COVID-19 illness may affect the menstrual cycle through the hypothalamic-hypophysis-ovarian-endometrial axis. Hypothalamic hypogonadism, occurring in COVID-19 and other severe diseases such as post-Ebola syndrome, explains long-term menstrual irregularity (9). The widespread expression of the ACE2 receptor in endometrial tissue provides an opportunity for SARS-CoV-2 to induce menstrual changes (3). The initiation of menstrual bleeding in the human uterus requires vasoconstriction of the spiral artery by Ang II, so a normal menstrual cycle depends on the accurate function of Ang II and its receptors in endometrial tissue. Any disturbance in this receptor expression leads to abnormal uterine bleeding (10, 11). Unfortunately, most large-scale COVID-19 investigations have ignored menstrual cycle changes, leaving questions about how many women experienced menstrual changes and the duration of these changes (12).
Anti-Müllerian hormone (AMH) is a glycoprotein expressed by granulosa cells of the ovarian growing follicles, and its serum level is a reference biomarker to evaluate ovarian reserve, oocyte quality, and quantity, and to predict reproductive aging. Unlike other reproductive hormones, AMH levels are not affected by hormonal fluctuations during the menstrual cycle (13).
2. Objectives
Due to the inconsistent and limited reports on the effects of COVID-19 on female fertility, especially in the post-COVID-19 recovery period, we designed this study to evaluate the long-term impression of SARS-CoV-2 infection on the menstrual cycle and AMH serum levels in women of reproductive age.
3. Methods
3.1. Study Design and Participants
This observational, single-center study was performed from March to September 2022, involving female patients (n = 103) of reproductive age (18 - 45 years) who were referred to the gynecology center at Kashani Hospital. Females with positive rapid antigen test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or positive COVID-19 rt-PCR test of throat swab samples were categorized as severe cases (n = 50) if they had hospitalization experience, oxygen therapy, a positive history of elevated respiratory rate (more than 30 breaths/min), or lung infiltration (more than 50%) (14). Non-severe or healthy age-matched females with no history of severe disease or any infection were considered the control group (n = 53). Participants who were three months or more post-infection were recruited to evaluate the long-term effects of COVID-19 on ovarian reserve and menstruation. We excluded women with a history of depression, psychiatric disorders, those undergoing hormone, anticoagulant, or chemotherapy, menstrual irregularity, or any history of infertility before the infection. Women diagnosed with polycystic ovary syndrome or endometriosis were also excluded to ensure the accuracy of our findings. Patients with cardiometabolic disorders, like obesity (BMI ≥ 35), hypertension, and type 2 diabetes, who had a higher risk of COVID-19 adverse consequences, and any patients who refused our request for blood sample collection were excluded from the study.
3.2. Data Collection Tools and Procedures
We designed a questionnaire in English based on the literature (12, 15) related to our topic, and then it was presented to experts in this field, such as obstetrics and infectious disease specialists, to validate its content. The questionnaire consisted of three sections: The first part addressed demographic data and the medical and obstetric history of the participants, while the second and third parts covered information related to COVID-19 and menstrual properties, respectively. Face-to-face interviews were conducted by three trained medical students who filled out the questionnaires after introducing themselves and the study subject.
The disturbances of the menstrual cycle that were evaluated included the severity of abdominal pain related to menstruation or dysmenorrhea, which was ranked into three types: No or mild/moderate; severe pain without any use of painkillers, enough to limit women’s activities; and severe pain controlled only with drugs or activity limitation within bleeding days. Menstrual abnormalities of cycle length and volume were classified as hypermenorrhea (menstrual flow of more than 7 days and 15 pads per cycle) or hypomenorrhea (menstrual flow of less than 3 days and 5 pads per cycle).
Plasma concentration of AMH was measured in the Nor Pathobiology Lab using an Ultra-Sensitive Ansh Labs immunoassay ELISA/MIS kit (AL-105-i), with normal range values of 0.08 - 14.2 ng/mL. An expert laboratory staff member received 2 mL venous blood samples from eligible participants, which were then centrifuged, and the serum was preserved at -30°C until all samples were collected. Then, 200 μL of serum was used to measure AMH concentration, and the rest of the serum was stored. The blood samples could be collected at any time of the menstrual cycle due to minimal fluctuation of AMH throughout the cycle; no fasting was required for this test.
3.3. Statistical Analysis
All analyses were carried out with SPSS software version 23. The results were expressed as median, interquartile ranges (IQR), mean and standard deviation (SD) for continuous variables, while categorical variables were presented as counts and percentages (%). The Mann-Whitney U test (non-parametric) and Independent sample t-test were used for continuous variables. Differences between categorical variables were examined by Pearson's chi-squared test. Spearman’s correlation analyses were applied to dysmenorrhea severity, marital status, employment, education, and AMH levels. Statistical significance was considered at P-values of < 0.05.
3.4. Ethical Consideration
This study was approved by the Jiroft University of Medical Sciences Ethics Committee (approval no. IR.JMU.REC.1400.052). Participants were requested to sign written consent before completing the survey, and they were assured that the information provided would remain confidential.
4. Results
125 women completed the questionnaire; however, 22 of them refused to give a blood sample, so they were excluded from the study. The remaining 103 participants were included in the final analysis. Tables 1 and 2 present their baseline characteristics. Women in case and control groups were 46% vs. 37.73%, 34% vs. 45.28%, and 18% vs. 16.98% for the 18 - 30, 31 - 40, and 41 - 50 years age distribution, respectively. The median age in the case and control groups was 31.50 years (IQR, 25.75 - 38.25) vs. 32.00 years (IQR, 26.00 - 39.00) (P = 0.65), respectively. The cases had a median BMI of 23.70 (21.25 - 27.72), which was similar to controls, 23.40 (21.85 - 26.30) (P = 0.49). There were no significant differences in terms of reproductive history indicators, gravidity, parity, or abortion between groups (P > 0.05) (Table 1). The level of education, marital status, and employment were similar in both cases and controls (P > 0.05) (Table 2).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Case (N = 50) | Control (N = 53) | ||
| Age (y) | 31.50 (25.75 - 38.25) | 32.00 (26.00 - 39.00) | 0.65 |
| 18 - 30 | 23/50 (46.00) | 20/53 (37.73) | |
| 31 - 40 | 17/50 (34.00) | 24/53 (45.28) | |
| 41 - 50 | 9/50 (18.00) | 9/53 (16.98) | |
| Body Mass index (BMI) | 23.70 (21.25 - 27.72) | 23.40 (21.85 - 26.30) | 0.49 |
| Reproductive history; mean ± SD | |||
| Gravidity | 2.32 ± 1.54 | 2.35 ± 1.48 | 0.89 |
| Parity | 1.88 ± 1.28 | 1.64 ± 1.24 | 0.34 |
| Abortion | 1.88 ± 1.28 | 0.64 ± 0.68 | 0.62 |
a Values are expressed as median (IQR) or No. (%) unless otherwise indicated.
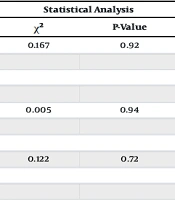
| Characteristics | Groups | Statistical Analysis | ||
|---|---|---|---|---|
| Case (N = 50) | Control (N = 53) | χ2 | P-Value | |
| Education | 0.167 | 0.92 | ||
| School level or below | 15 (30.00) | 16 (30.18) | ||
| Diploma | 25 (50.00) | 28 (52.83) | ||
| Bachelor degree | 10 (20.00) | 9 (16.98) | ||
| Occupation | 0.005 | 0.94 | ||
| Housewife | 27 (54.00) | 29 (54.71) | ||
| Employed | 23 (46.00) | 24 (45.28) | ||
| Marital status | 0.122 | 0.72 | ||
| Married | 30 (60.00) | 23 (43.39) | ||
| Non-married (single, divorced) | 20 (40.00) | 30 (56.60) | ||
Abbreviation: χ2, pearson chi-square value.
a Values are expressed as No. (%).
Comparisons of severe COVID-19 cases (n = 50) and controls (non-severe and healthy participants) (n = 53) in terms of the ovarian reserve marker, AMH serum levels, after an average duration of 4.92 ± 1.06 vs. 5.01 ± 1.11 months (P = 0.95) since infection in the case and controls respectively, show significant decreases in mean serum levels of AMH in the case compared to controls (2.66 ± 1.95 vs. 3.38 ± 2.59 ng/mL, P = 0.03), as presented in Table 3.
| Variables | Groups | Statistical Analysis | ||
|---|---|---|---|---|
| Case (N = 50) | Control (N = 53) | t-Value | P-Value | |
| Anti-müllerian hormone (ng/mL) | -1.57 | 0.03 | ||
| Mean ± SD | 2.66 ± 1.95 | 3.38 ± 2.59 | ||
| Median (IQR) | 2.13 (1.06 - 4.25) | 2.80 (1.10 - 4.99) | ||
| Duration since COVID-19 infection (mon); mean ± SD | 4.92 ± 1.06 | 5.01 ± 1.11 | 1.20 | 0.95 |
Table 4 shows the comparison of menstrual abnormalities between cases and controls in the post-COVID-19 recovery period. Thirteen (26.00%) women in the case group and 15 (28.30%) in the control group reported hypermenorrhea (P = 0.79, χ2 = 0.06) in the last menstrual cycles. In terms of hypomenorrhea, no significant differences were observed between severe COVID-19 infected cases and controls, 7 (14.00%) vs. 8 (15.10%) respectively (P = 0.64, χ2 = 0.21). Despite no significant differences in abnormalities related to menstrual cycle length and volume between groups, dysmenorrhea was more prevalent in cases than controls, 26 (52.00%) versus 17 (32.07%) respectively (P = 0.02, χ2 = 5.05). There were no significant differences in the mild form of dysmenorrhea between groups, while moderate and severe forms were more obvious in cases than controls (Table 4).
| Menstrual Disturbance | Groups | Statistical Analysis | ||
|---|---|---|---|---|
| Case (N = 50) | Control (N = 53) | χ2 | P-Value | |
| Hypermenorrhea | 13 (26.00) | 15 (28.30) | 0.06 | 0.79 |
| Hypomenorrhea | 7 (14.00) | 8 (15.10) | 0.21 | 0.64 |
| Dysmenorrhea | 26 (52.00) | 17 (32.07) | 5.05 | 0.02 |
| Mild | 14 (28.00) | 14 (26.41) | ||
| Moderate | 8 (16.00) | 2 (3.77) | ||
| Severe | 4 (8.00) | 1 (1.88) | ||
a Values are expressed as No. (%).
Nevertheless, the more severe dysmenorrhea perceived by females recovered from severe COVID-19 was not related to factors including marital status (r = 0.10, P = 0.27), employment (r = 0.16, P = 0.10), education (r = 0.11, P = 0.24), and AMH serum levels (r = 0.03, P = 0.74) (Table 5).
| Variable | Marriage | Employment | Education Levels | AMH Levels (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| r | P-Value | r | P-Value | r | P-Value | r | P-Value | |
| Dysmenorrhea severity | 0.10 | 0.27 | 0.16 | 0.10 | 0.11 | 0.24 | 0.03 | 0.74 |
Abbreviation: AMH, anti-Müllerian hormone.
5. Discussion
The study was conducted to analyze the long-term effects of SARS-CoV-2 infection on the ovarian reserve and menstrual changes of reproductive-aged women in the post-COVID-19 recovery period. Our results revealed a significant reduction in the ovarian reserve of participants with a positive history of severe COVID-19. Comparison of menstrual abnormalities between cases and controls disclosed similar results regarding menstrual volume and cycle length abnormalities; however, the severity of menstrual pain was significantly higher compared to the controls.
Based on AMH levels analyses in the present study, severe COVID-19 causes a reduction in the ovarian reserve during the post-COVID-19 recovery period, which is consistent with Yousif et al. in 340 Iraqi fertile women with a positive history of severe COVID-19. They revealed a decrease in ovarian reserve indicators, AMH, and antral follicle count during the post-infection course compared to age-matched controls (16). However, this effect wasn’t observed by El-Samie et al. in a cross-sectional survey of 120 Egyptian infertile women that were in the duration of 5 months post-COVID-19 infection. Seventy-five percent of participants reported a positive history of a mild form of the disease, and only 25% mentioned a severe form. The comparison of serum levels of AMH before and during the post-COVID-19 period didn’t show a statistically significant difference (17). Also, an observational study conducted by Madendag et al. on 132 infected Turkish women of reproductive age, before and during the post-COVID-19 recovery period, reported no significant effect on AMH serum levels. Only 2.3% of participants revealed a positive history of severe disease; the rest had a positive history of mild diseases (18). This controversy can be explained by different study methodologies and the low rate of participants with a history of severe COVID-19 in their study. Declines in the ovarian reserve have been detected after some viral infections, including hepatitis B and C and human immunodeficiency virus; nonetheless, insufficient evaluations have been conducted on COVID-19-induced effects on the female reproductive system (19, 20). Probably systemic inflammation induced by the viral infection adversely impacts ovarian granulosa cells, reducing the production of AMH. The psychological stress during the COVID-19 pandemic leads to elevated stress hormone levels that induce destructive effects on granulosa and theca cells of ovarian follicles. These endocrine dysfunctions cause decreases in serum AMH levels (21, 22).
Anti-Müllerian hormone has multiple physiologic roles in the ovary. The number of primordial follicles that exist in the ovary as follicular stock or ovarian reserve is reflected by the number of growing follicles that produce AMH as a prime marker for estimating the ovarian reserve (23). On the other hand, AMH in a paracrine manner inhibits primordial follicle recruitment, thus preserving the ovarian reserve by stopping their activation (24). Therefore, further exploration of AMH levels in women who recovered from COVID-19 is crucial to provide a supportive fertility plan.
Some previous studies claimed that women of reproductive age infected by COVID-19 experienced changes in their menstruation. Major changes recorded included alteration in menstrual patterns, increased or decreased menstrual volume, prolonged cycles, and increased episodes of pain (25, 26). Our study showed that during the post-COVID-19 recovery period, menstruation volume and cycle length abnormalities were not significant in cases compared to controls, while significant menstrual pain was detected. Deogade et al. conducted a cross-sectional observational study on 47 reproductive-age women. They obtained women’s information telephonically from an Indian COVID health center. The participants were interviewed in the third month after discharge, and they found that post-COVID-19 menstrual irregularity, abnormal duration, and menstruation volume were not statistically significant compared to pre-COVID-19 history; only the menstrual pain score was significant (15). Also, Aolymat et al. conducted a cross-sectional survey of 385 medical students to evaluate the impact of the COVID-19 pandemic on dysmenorrhea and PMS (premenstrual syndrome) symptoms. Their results showed COVID-19-associated depression, anxiety, and stress scores were positively related to PMS components and dysmenorrhea(27). According to a digital survey of 1031 women during the pandemic, conducted by Phelan et al., 49% of participants expressed painful periods, and in 7% of them, a higher incidence of dysmenorrhea was observed (28). Maher et al. recruited 1335 fertile women in a cross-sectional online study to evaluate the long-term COVID-19 effects on the reproductive and mental health of selected participants. Their results revealed that mental health disturbances with symptoms such as anxiety, stress, low mood, and loneliness, as well as the incidence of dysmenorrhea, heavy periods, and missed periods, significantly increased during the pandemic. An increase in anxiety levels leads to the conversion of non-painful menstruation into a painful type (29). During the COVID-19 pandemic, psychological, interpersonal, and environmental stressors induced negative effects on the regulation of the hypothalamic-pituitary-gonadal axis, consequently inhibiting the release of Gonadotropin-releasing hormone (GnRH). Higher cortisol levels inhibit LH secretion, leading to ovarian steroidogenesis suppression and abnormal fluctuations of menstrual regulatory hormones (30, 31). Coronavirus disease 2019, as a pro-inflammatory disease, generates cytokine storms and immune exhaustion. Post-COVID-19 dysmenorrhea can probably be attributed to such inflammatory changes (32).
5.1. Limitation
The present study had some limitations. Firstly, only 103 participants from a single center were used to run the survey, while a larger sample size and multi-center evaluation are needed for further conclusions. Receiving the menstrual characteristics through the interview was subjective and could cause misinterpretation; nevertheless, we used face-to-face interviews to overcome this problem.
5.2. Conclusions
Our observational study suggested that a positive history of severe COVID-19 is significantly related to a decrease in serum AMH levels, as an ovarian reserve marker, during the post-recovery period of COVID-19 in reproductive-age women. Despite more severe dysmenorrhea, the abnormality of menstrual volume and cycle length was not significant when compared to the non-severe and uninfected controls. Given the controversial results regarding the female reproductive system in the post-COVID-19 recovery period, more comprehensive research with a larger sample size is needed to confirm the long-term effects of COVID-19 on female fertility.