1. Background
Patellofemoral pain syndrome (PFPS) is one of the most prevalent conditions among physically active individuals, with a higher incidence among females (13 - 26%) (1, 2). The etiology of PFPS is multifactorial and interrelated (3); however, the importance of hip and core stability in assessing and managing patients with PFPS has been emphasized (4).
The lumbo-pelvic-hip complex (LPHC) includes all contractile and non-contractile elements of the middle trunk with the associated controlling nervous system (5, 6). Muscles in the LPHC may contribute to the stability of the trunk and limbs in a kinematic chain by providing a foundation for controlling motions and forces during activities of daily living (7). The diaphragm, as an internal part of this system, along with the anterior abdominal and pelvic floor muscles, adjusts internal abdominal pressure to maintain both dynamic and static stability (8, 9). Additionally, core stability is maintained by the efficient function of the lumbo-pelvic-hip complex to control buckling and maintain stability after inducing perturbation.
Moreover, LPHC stability, as a remote factor, is proposed to have impacts on knee joint alignment and function (10). Previous studies have shown that movements of the trunk in the frontal and transverse planes can directly impact the frontal and internal rotation moment of the knee, respectively (11, 12). Therefore, deficiencies in the stability of the LPHC may impair the stability of the entire kinematic chain, especially in the frontal plane, particularly knee joint stability, and consequently may exacerbate PFPS symptoms (4, 13). Recently, LPHC deficiencies, such as abnormal muscle recruitment patterns (6) and decreased hip and trunk muscle strength (14), were observed in patients with PFPS.
Furthermore, the diaphragm, as the primary respiratory muscle, along with the pelvic floor and trunk muscles, is regarded as an integral part of the LPHC system, contributing to core stability (15, 16). Working in conjunction with the anterior abdominal wall muscles, the diaphragm also influences intra-abdominal pressure (17). It has been proposed that the connections between the diaphragm muscle, thoracolumbar fascia, and transverse fascia serve as pathways for transmitting force and information between the abdominal cavities and the chest, thereby enhancing LPHC strength and stability (18). Consequently, changes in motor control of this muscle may impact the entire motor control chain, including the knee joint (19). Numerous studies have explored the role of the diaphragm muscle in dysfunctions of the shoulder (20), pelvis (21), ankle (22), and lower back (23). One study found that compromising core stability by inducing fatigue in both superficial and deep core musculature in novice female runners led to an increase in peak knee flexion moment during stance, a major risk factor for developing PFPS (13). Additionally, recent research has shown that incorporating core stability exercises into routine exercise regimens can improve knee pain and function in individuals with PFPS (4). Therefore, considering the dearth of studies investigating the function of the diaphragm muscle as a potential contributing factor and core element in exacerbating PFPS, as well as the importance of trunk stability in these patients, this study aimed to compare the endurance of trunk muscles, representing LPHC stability, and the contractility of the diaphragm muscle in females with PFPS and healthy females.
2. Objectives
Considering the lack of studies examining the functions of the diaphragm muscle as a possible contributing factor in exacerbating PFPS and the importance of trunk stability in these patients, this study aimed to compare the endurance of trunk muscles, representing LPHC stability, and the contractility of the diaphragm muscle in females with PFPS and healthy females. The study hypothesis was that there would be differences in trunk muscle endurance and diaphragm muscle contractility in females with PFPS.
3. Methods
Fifty-six females aged 15 - 45 years participated in this study. The PFPS group (experimental group) comprised 28 females diagnosed with PFPS, while the control group included 28 subjects without a history of PFPS. The sample size was calculated based on a pilot study involving 16 females with and without PFPS, using the mean and standard deviation obtained for the endurance scores of trunk extensors. Power-G software with a power of 95% and α = 5% was utilized to estimate the sample size.
The two groups were matched based on weight, height, and physical activity level using the Persian version of the Tegner activity level questionnaire (24). This case‒control study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1396.473). All participants provided written consent before the study. Data collection took place at the Biomechanics Research Center of Tehran University of Medical Sciences from 2018 to 2019.
3.1. Inclusion Criteria
For the PFPS group, inclusion criteria were as follows: Diagnosis of patellofemoral pain syndrome by an orthopedic specialist in one knee, along with presenting pain and limitations during activities such as running, jumping, descending and ascending stairs, and prolonged sitting (3, 25), for at least the last three months, and a body mass index (BMI) in the range of 18.5 < BMI < 25. The most painful knee was evaluated in cases of bilateral knee pain. The control group had no pain or functional limitations of the knee joint.
3.2. Exclusion Criteria
Exclusion criteria for both groups included any history of pain in the lumbar, hip, or ankle regions, experiencing any vestibular or balance disorder, concussion, or other head injuries in the last six months, a history of surgery in the lumbar or lower limbs, any cardiorespiratory disease, ankylosing spondylitis, or any other disorder based on medical history that impacts lower limb or body performance, or any musculoskeletal or neuromuscular disease other than PFPS (4, 26).
3.3. Lumbo-Pelvic-Hip Endurance Tests
Endurance tests were conducted following the McGill method (27), which has been shown in previous studies to be valid (28) and reliable (ICC = 0.91 - 0.98). Due to the fatigue-inducing nature of these tests, a two-minute rest interval was provided between trials. All tests were randomly performed, with a 3-minute interval between each test.
3.4. The Trunk Extensor Muscles Endurance Test
This test was employed to assess the posterior muscles of the LPHC. Participants were instructed to lie prone with their hips, knees, and lower limbs fixed on the examination table while placing both hands on the chair and keeping the trunk off the table. The examiner measured the endurance time as long as the body remained straight and recorded the Extensor Muscles Endurance Test (EET) (27).
3.5. Trunk Flexor Muscle Endurance Test
Participants started in a supported 60-degree sit-up position with both knees at 90 degrees and feet fixed to the ground. After removing the support, they maintained the position as long as possible. The test continued until the participant could no longer maintain the position, and the examiner recorded the endurance time as the Flexor Muscle Endurance Test (FET) (27).
3.6. Trunk Right Side Muscles Endurance (Side Bridge) Test
This test assessed the stability of the lateral side of the body. Participants began in a side-lying position with straight knees, with the top foot fixed in front of the lower foot. They lifted their hip off the table, supporting their weight only on the elbow and feet. The examiner recorded the time as long as the participant maintained the position (23). The test was performed on both sides (27).
To measure LPHC endurance, the endurance of the FET, Right Side Muscles Endurance (Side Bridge) Test (RSBT), and LSBT was normalized to the EET. Therefore, the endurance ratios of FET/EET, RSBT/EET, and LSBT/EET were calculated. The normal values for FET/EET, RSBT/EET, and LSBT/EET are 1/3 (0.33) and 1/2 (0.50), respectively (27). Achieving the normal value indicated a positive test, signifying that muscle performance could maintain the equilibrium of the interacting pillars of the LPHC, thereby stabilizing the PHC.
The severity of knee pain was assessed using a visual pain scale to measure pain experienced during daily activities over the last three months.
Diaphragm muscle contractility was examined using type B ultrasound with an electronic Honda H 2600 ultrasound device, focusing on the zone of opposition between the eighth and ninth ribs (29). Among the available methods for ultrasound analysis of the diaphragm muscle, the supine position method has demonstrated acceptable reliability. Additionally, inspired volume and diaphragm excursion have been found to correlate in this position (30). Therefore, during three slow breaths, the thickness of the diaphragm muscle on both the left and right sides was measured. This measurement was taken at the end of inspiration, when the muscle is thickest and shortest, and at the end of exhalation, when the muscle returns to its resting state due to eccentric contraction. The distance was recorded in centimeters, and the average of three trials was used as the diaphragm thickness (31). The mean diaphragm thickness from the three slow breaths was used for analysis.
Subsequently, the percentage of diaphragm muscle contraction was calculated using the following formula, which has been deemed more reliable than comparing muscle thickness at the end of inspiration:
[(Inspiration thickness - expiration thickness)/Expiration thickness] × 100
3.7. Data Analysis
Out of the 89 individuals referred to the treatment center, 37% were excluded from the study due to their inability to perform endurance tests in both groups. These individuals were unable to complete the study tests or their modified versions, or they experienced severe pain in the neck and back that hindered them from maintaining the required position during the endurance tests.
The data were analyzed using SPSS Version 24 at a significance level of 0.05. The Kolmogorov‒Smirnov test was applied to assess the normality of the data. The Mann‒Whitney U test was utilized to compare the means of the endurance tests, diaphragm contractility, and demographic data between the two groups. The one-sample t-test was employed to evaluate differences in BMI between the two groups.
4. Results
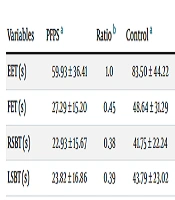
The demographic characteristics of the participants are presented in Table 1. According to the Tegner activity level questionnaire, the activity levels of participants in the patient and control groups were 2 (35.7%) and 3 (35.7%), respectively. Significant differences exist in the trunk muscle endurance and endurance ratios between the two groups. The endurance of trunk muscles was lower in the PFPS group than in the healthy group (P < 0.05) (Table 2).
| Variables | PFPS Group | Control Group | P-Value |
|---|---|---|---|
| Age | 36.29 ± 8.52 | 35.82 ± 6.08 | 0.283 |
| Weight | 58.32 ± 7.90 | 57.57 ± 6.11 | 0.605 |
| Height | 162.39 ± 6.08 | 162.32 ± 4.98 | 0.761 |
| Body Mass Index (BMI) | 22.04 ± 2.29 | 21.72 ± 1.90 | 0.564 b |
| Visual Analog Scale | 3.00 ± 1.00 | 0.00 ± 0.00 | 0.000 |
Demographic Characteristics of Participants in the Two Groups a
Comparison of Mean Endurance Time and Normalized Ratios between the Two Groups
Descriptive statistics and the distribution of diaphragm-related variables in the two groups are presented in Table 3. According to the results, patients with PFPS exhibited weaker contractility and thickness of the diaphragm muscle on the right side compared to the control group.
| Variables | Healthy (n = 28) | PFPS (n = 28) | P-Value | Effect Size |
|---|---|---|---|---|
| Left inhalation diaphragm thickness | 0.23 ± 0.03 | 0.21 ± 0.03 | 0.36 | 0.11 |
| Left exhalation diaphragm thickness | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.33 | 0.14 |
| Percentage of left diaphragm contraction | 27.03 ± 7.07 | 22.92 ± 2.99 | 0.00 c | 0.16 |
| Right inhalation diaphragm thickness | 0.24 ± 0.03 | 0.23 ± 0.03 | 0.00 c | 0.37 |
| Right exhalation diaphragm thickness | 0.18 ± 0.03 | 0.18 ± 0.02 | 0.37 | 0.12 |
| Percentage of right diaphragm contraction | 32.12 ± 10.55 | 21.82 ± 3.61 | 0.00 c | 0.64 |
5. Discussion
The main objectives of this study were to compare the endurance of the lumbar-trunk-pelvic muscles in the PFPS and non-PFPS groups and explore the difference in the function of the LPHC as a unified system, focusing on the diaphragm muscle as an integral part of this complex. The results showed a significant difference in endurance time in all muscle groups in the PFPS group. Patients in the PFPS group demonstrated lower endurance times in the bilateral muscles of the LPHC and weaker contractility in the right diaphragm.
The findings revealed that endurance is impaired in all four muscle groups of the LPHC, with the lowest rate observed in both lateral muscle groups in females with PFPS. Previous studies have provided conflicting evidence regarding LPHC endurance in patients with PFPS. While some studies showed no difference in LPHC endurance in patients with PFPS (32, 33), others suggested an LPHC endurance deficiency in the PFPS group (34, 35). Ferber et al. investigated the effectiveness of a 6-week "knee" or "hip and knee" strengthening program in managing PFPS. Regardless of the grouping of the subjects into the knee or hip and knee protocol, improvements in pain (VAS and Anterior Knee Pain Scale), strength, and endurance were observed in patients (36). Some other studies reported no significant difference between PFPS and healthy groups. However, these studies had a small patient cohort or examined male and female participants regardless of gender differences, which has made their findings of little credibility (32, 33).
As mentioned earlier, there were significant differences in side trunk endurance on both sides between groups. The activity of the external abdominal oblique and quadratus lumborum muscles during the side bridge test is accompanied by the activation of the Gluteus medius muscle (37, 38). Strength deficits in the gluteus medius muscle in PFPS patients have been reported (37). Therefore, the differences in side trunk muscle endurance between people with PFPS and healthy subjects may be affected by the decreased stabilizing effect of the gluteus medius muscle on the knee joint in the frontal plane in people with PFPS. Cowan et al. studied the stabilizing act of the Gluteus medius and reported a delay and lower activity of the Gluteus medius during the ascending stair task (7).
In this study, there was a statistically significant difference between the two groups in the inhalation thickness of the right half of the diaphragm muscle. Additionally, a statistically significant difference was found in the contraction percentage of the right and left sides of the diaphragm muscle. These values were higher in the healthy group. Additionally, the difference in expiratory thickness of the diaphragm in the two groups was not significant. To control for confounders, the two groups were matched in terms of age, body mass index, height, and level of physical activity. Therefore, having no difference in muscle thickness can be due to the similarity of the two groups in terms of the factors mentioned above and the initial level of activity of the diaphragm muscle.
According to the results of this study, the differences in the right and left sides of the diaphragm muscle in the two groups can indicate a change in muscle function and a shift in the pattern of respiratory function in PFPS. Hodges and Gandevia studied diaphragm muscle activity when applying resistance to the lower limb and found tonic activity independent of the respiratory activity of the diaphragm muscle but in harmony with the frequency of activity of the main muscles moving the limb (39).
As observed in our study, the uncoordinated function of the two sides of the diaphragm muscle in females with PFPS may be due to the increased valgus angle of the knee, which in the kinematic chain could be associated with increased lateral pelvic oscillation (39). The higher percentage of diaphragm muscle contraction on the left side in the healthy group compared to the PFPS group may indicate decreased diaphragm muscle function in PFPS. Since the left side of the diaphragm muscle regulates and transmits forces by regulating the intra-abdominal pressure and creating a strong base in the middle of the body, it can be concluded that in females with PFPS, the function of the diaphragm muscle in creating stability in the trunk and pelvis is reduced. However, in our study, alternative patterns for maximum stability were not investigated.
5.1. Conclusions
This study showed that LPHC endurance decreased in all four sides of trunk muscles, with a higher reduction observed in bilateral sides and low contractibility in the right diaphragm muscle. Future studies should assess the integrity of the diaphragm muscle and the spirometry of respiratory capacity simultaneously. Additionally, checking the onset of muscle activity by type M ultrasound or electromyography would be helpful.
5.2. Limitation
Not considering the structural deviations of the knee like Q angle and structural malalignments like femoral anteversion or knee valgus in the inclusion criteria for patients with PFPS were important limitations of this study. The results of this study are limited only to females with PFPS, not men with PFPS.