1. Background
Parkinson's disease (PD) is a neurodegenerative disorder characterized by motor impairments such as rigidity and bradykinesia, as well as non-motor impairments. These impairments can have a negative impact on activities, participation, and overall quality of life for individuals with PD (1).
Difficulties with activities of daily living (ADLs) are frequently the primary reason individuals seek medical consultation and ultimately receive a diagnosis of PD. The impact of PD on ADLs has been extensively studied (2). Inconsistent findings in PD research can be attributed to the specific nature of the coordination tasks being assessed (3). Parkinson's disease is known to pose significant challenges in coordinating movements involving synergistic interactions between muscles or limbs. The coordination process involves complex neural integration at both high and low levels, occurring temporally and spatially (2, 4). Bimanual coordination function in PD can become more disrupted as the disease progresses (3, 4). Many ADLs require both hands to work in coordination, performing tasks involving the use of objects and tools. Activities such as eating, bathing, grooming, and various household chores often require the use of both hands to manipulate objects, utensils, or tools effectively (5). It is essential to consider the challenges that individuals with PD may face in performing these activities.
Functional-task training involves engaging in exercises that simulate daily activities, incorporating different attributes and interactions with others in various environments. The purpose of these interventions is to promote individuals' movement capabilities, known as motor learning (6).
There is evidence to indicate that PD is associated with a common deficit in motivation, which can affect multiple aspects of motivation (7). Motivation can be defined as a conscious or unconscious internal state that drives an individual to take action (8). Motivational changes in PD are less well understood compared to motor symptoms. However, it is widely acknowledged that these changes can be some of the most debilitating symptoms of the disease (3). One model of occupational therapy (OT) that emphasizes the effect of motivation on performance improvement is the model of human occupation (MOHO). In this model, motivation is defined as the desire to perform occupation (9). In a study examined daily activities using the MOHO model, they found that performing activities using this model significantly differed from performing activities without using the model (10).
Due to these limitations, there may be a gradual decline in one's ability to perform functional tasks and maintain an independent lifestyle (11, 12). Both physical therapy (PT) and OT share the common goal of enhancing functional autonomy and participation. The primary focus of occupational therapists is to empower patients by promoting their performance and participation in significant activities in their homes and communities (13). Given the importance of activity-based interventions and the lack of research on evaluating such interventions on the upper extremities in these patients, along with the absence of studies on combining activity-based interventions with motivational strategies - both core concepts in OT, there is a need to conduct and assess a randomized controlled trial (RCT) focusing on these specific areas.
2. Objectives
This trial study aims to investigate the impact of bimanual activities on participation and sensory-motor skills in individuals with Parkinson's disease. The study will apply three different interventions: (1) bimanual activity-based intervention with a motivational strategy; (2) bimanual activity-based intervention without a motivational strategy; (3) conventional interventions.
3. Methods
3.1. Trial Design
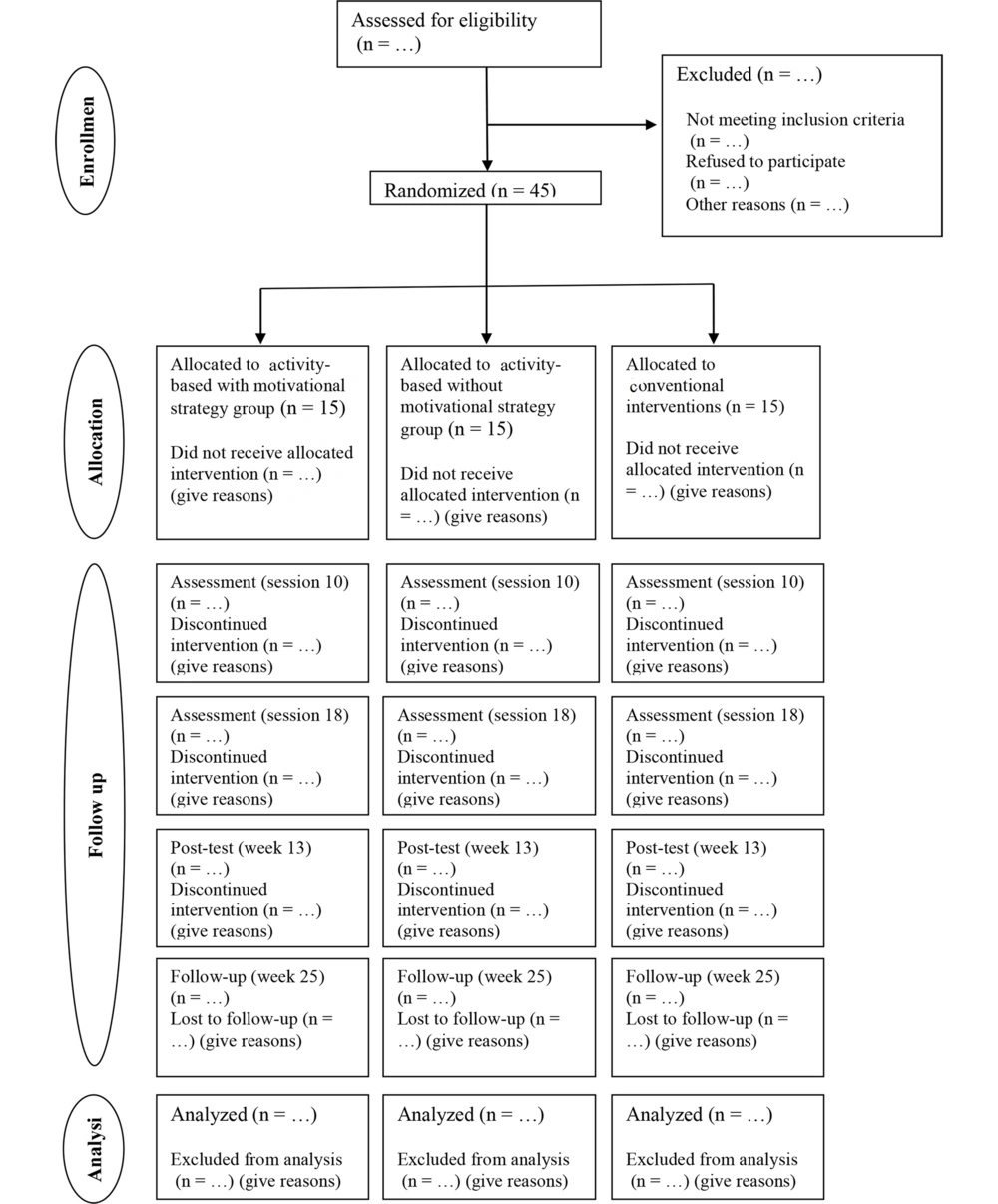
This research is a clinical trial that follows a double-blinded controlled randomized trial with parallel groups. The study aims to investigate the effects of different interventions on PD. A total of forty-five patients with PD will be enrolled in this study, with an equal distribution ratio of 1:1:1 among the three groups. The participants will be randomly assigned to one of the following three groups: A control group, bimanual activities with motivational strategies, and bimanual activities without motivational strategies. To provide a concise overview of the trial design, the CONSORT diagram (14) (Figure 1) will depict the process of enrollment, allocation, and assessments.
3.2. Participants and Recruitment
Patients diagnosed with PD will be selected from rehabilitation and outpatient clinics situated in Tehran, Iran. Those patients who meet the inclusion criteria, as outlined in the section below, will be provided with a consent form and comprehensive information regarding the objectives and procedures of the study. All sessions will be offered to participants free of charge, and any expenses incurred for travel during evaluations will be covered by the research team. Both assessments and interventions will be carried out in the "ON" state of patients, which is approximately one hour after taking their levodopa medication. To determine the levodopa equivalent dose (LED) for each individual in every assessment session, the Tomlinson et al. formula (15) will be utilized.
3.3. Data Collection
An experienced occupational therapist with a Master's degree in OT, who has over 5 years of experience in neurological rehabilitation, will perform an initial screening and evaluate the outcomes of intervention at baseline, session 10, session 18, post-intervention (week 9), and follow-up (week 17) (Table 1). Other therapists will conduct the interventions.
| Enrollment | Allocation | Treatment Period | Post-Intervention | Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time-points | T-1 | T0 | T1 | T2 | … | T23 | T24 | T25 | T51 |
| Enrollment | |||||||||
| Eligibility | √ | ||||||||
| Screening | √ | ||||||||
| Informed consent | √ | ||||||||
| Randomization | √ | ||||||||
| Intervention | |||||||||
| Group A | → | ||||||||
| Group B | → | ||||||||
| Group C | → | ||||||||
| Assessments | |||||||||
| Baseline | Session 10 | Session 18 | Post Intervention | Follow-up | |||||
| Demographic and PD-related | √ | ||||||||
| Control of upper extremity | √ | √ | √ | √ | √ | ||||
| Sensory-motor skills of upper extremity | √ | √ | √ | √ | √ | ||||
| Participation | √ | √ | √ | √ | √ | ||||
| Cognitive function | √ | √ | √ | √ | √ | ||||
| Quality of life | √ | √ | √ | √ | √ | ||||
a Group A, control group; group B, bi-manual activities with motivational strategies; group C, bi-manual activities without motivational strategies.
3.4. Inclusion and Exclusion Criteria
The inclusion criteria will be: Diagnosis of PD by a neurologist (based on the UK Brain Bank criteria for the diagnosis of idiopathic PD) (16), progression level of 1 to 3 according to the Hoehn and Yahr Scale (17), acceptable level of cognitive function (i.e., a score equal to or greater than 24 on the Persian version of the Mini-Mental State Examination (18), and a score greater than 24 on the Persian version of the Montreal Cognitive Assessment Questionnaire) (19), at least a fifth-grade education, ability to perform uni-manual and bi-manual motor tasks, feeling the need or desire to participate in occupations selected for the intervention (bi-manual occupations in the form of ADL, instrumental ADL, and playing).
The exclusion criteria will be co-morbid other neurological diseases, orthopedic disorders (such as low back pain and arthritis), diabetes or addiction according to the report of the patient, their family, or physician, pathological dementia according to the neurologist's diagnosis, and Juvenile Parkinsonism.
3.5. Randomization, Blinding, and Ethics
The intervention protocol received approval from the university's ethics committee (IR.IUMS.REC.1398.794). After obtaining consent, patients included in the study will be randomly allocated to one of the following three groups: (A) the control group; (B) the bi-manual activities with motivational strategies group; or (C) the bi-manual activities without motivational strategies group. The randomization method is simple randomization, and the randomization unit is individual. Randomization and the creation of a random sequence will be conducted using a web-based randomization system (https://www.graphpad.com/quickcalcs/randMenu/). Group allocation concealment will be ensured using sequentially numbered sealed envelopes by statisticians who are not part of the project team and will be blind to any interventions implemented. Evaluation of the primary and secondary outcomes will be performed by occupational therapists experienced in neurological diseases, especially PD, who are blind to the group allocations. To maintain assessor blinding, participants and therapists will be instructed not to disclose the received intervention to the assessors. Patients will also be blinded to the group allocations, and interventions and evaluation sessions for different groups will be scheduled to prevent encounters between participants in different groups.
3.6. Sample Size Calculation
G-Power software was used to estimate the sample size for the study. The standardized effect size (Cohen's f) was set at 0.25, with a power of 80% and a type I error rate of 5%, all based on the primary outcome measure, the Utrecht Scale for Evaluation of Rehabilitation Participation Satisfaction (20). Considering a projected dropout rate of 10%, the total sample size was calculated to be 45 subjects. To ensure the adequacy of the sample size, a power calculation will be conducted again at the conclusion of the trial.
3.7. Interventions
The significance of therapy for individuals with PD will be elucidated to the participants at the beginning. Treatment sessions will be administered three times a week for a duration of 24 weeks in each group. Each session is expected to last approximately 90 minutes. The timing of these sessions, either in the morning or evening, will be based on the participant's status and medication schedule to ensure they are in an "ON" state during treatment. Participants will rest as needed, and they will be instructed not to engage in any other forms of rehabilitation intervention during treatment. Additionally, the intervention will be discontinued upon the expressed choice of participants to terminate their involvement. Both intervention programs for activity-based intervention and motivational strategy were discussed in a panel of 10 experts, including occupational therapists, physical therapists, and neurologists, each with at least 5 years of experience in PD rehabilitation and intervention.
3.7.1. First Intervention Group: Activity-Based Intervention with Motivational Strategy
The bi-manual activities with motivational strategies group will receive 24 sessions of intervention (8 weeks, 3 sessions per week, 90 minutes per session) involving bi-manual activities, including four ADL, instrumental ADL, and playing, according to the Gentile theory and taxonomy for upper extremity, alongside conventional rehabilitation. This group will utilize various motivational strategies, such as affirmations to foster a sense of usefulness, facilitating fun by enhancing the likelihood of success, providing appropriate positive feedback to increase the patient's awareness of their abilities, demonstrating positive changes in performance during intervention sessions, and explaining the importance of the activity to the individual and its impact on daily life (see Appendices 1-3), based on the MOHO of OT.
3.7.2. Second Intervention Group: Activity-Based Intervention without Motivational Strategy
The bi-manual activities without motivational strategies group will undergo 24 sessions of intervention (8 weeks, 3 sessions per week, 90 minutes per session) involving bi-manual activities, including ADL, instrumental ADL, and playing, in addition to conventional rehabilitation. Unlike the first group, motivational strategies such as affirming the patient to facilitate a sense of usefulness, helping to gain a sense of fun by facilitating the likelihood of success, giving appropriate positive feedback to increase patient's awareness of his/her abilities to perform the activity, showing positive changes in patient's performance during intervention sessions and explain the importance of the activity to the individual and how it affects his/her daily life) will not be employed in this group.
3.7.3. Conventional Interventions
Participants assigned to this group will exclusively receive a total of 24 sessions of conventional treatment over 8 weeks, with 3 sessions per week lasting approximately 90 minutes each. The treatment will encompass a range of therapeutic activities, such as passive mobilization, stretching exercises, motor coordination exercises for the upper extremities, manipulation exercises, breathing exercises, relaxation techniques, and postural exercises (21, 22). The specific interventions provided to each patient will be tailored based on their individual condition.
3.8. Measurements
Participants will be asked to provide various demographic and PD-related details, including age, gender, level of education, family and marital status, employment status, use of assistive devices, history of falls, time since diagnosis, and medications taken. To evaluate the effectiveness of the intervention, a certified occupational therapist, unaware of the participants' conditions, will assess outcome measures at multiple time points: Before the intervention (baseline), after the 10th session, after the 18th session, immediately post-intervention, and during the follow-up period. Patients will complete the questionnaires in a peaceful and suitable setting. If they begin to feel exhausted, they will be given the opportunity to take a break between assessments. The inquiries will be conducted over two separate sessions.
3.8.1. Primary Outcome Measure
3.8.1.1. Upper Extremity Control
To achieve this objective, we will use a motion analysis system (Vicon 2.5, with a sampling frequency of 250 Hz) (23) (Appendix 4 in the Supplementary File).
3.8.1.2. Sensory-Motor Skills of Upper Extremity
For this purpose, we will utilize the wrist position sense test (24)and hand active sensation test (25) to assess sensory function. Additionally, we will employ the box & block test (26), grip dynamometer test (27, 28), Purdue Pegboard test (29), Nine-Hole Peg test (30, 31), and coin rotation test (32) to evaluate fine and gross motor function.
3.8.1.3. Participation
To assess patient participation, we will use the Canadian occupational performance measure (33, 34) and the Meaningful Activity Participation Assessment Questionnaire (35).
3.8.2. Secondary Outcome Measures
3.8.2.1. Cognitive Function
To assess cognitive function, we will use a set of various tests, including the clock drawing test (36), Parkinson's Disease-Cognitive Rating Scale (37), trail making test (38, 39), Stroop test (40), Quick Dementia Rating Scale (41), digit symbol test (42), and Benton test (43). Through these evaluations, we aim to gain insights into the patient's cognitive abilities and determine their level of cognitive functioning.
3.8.2.2. Quality of Life
Quality of life assessment will utilize two questionnaires: The PD Questionnaire (PDQ-39) (44) and the 36-item short form health survey (SF-36) (45). The aim is to evaluate the impact of PD on an individual's overall well-being.
3.9. Data Analysis
Each participant will be assigned a unique registration number. All questionnaires and datasets will be securely saved in separate lockers and computer systems. In case of missing data, random coefficient analysis (46) will be used to handle it. Intention-to-treat analysis will be conducted for participants who drop out during treatment sessions or follow-up assessments. These dropout participants will still be included in the sample size and data analysis. The trial does not include any planned interim analyses or stopping rules.
For quantitative variables, the mean (standard deviation) or median (interquartile range) will be calculated. Categorical variables will be presented as frequency (percentages). To assess the normality of the data, the Shapiro-Wilk test, graphical methods, and numerical indices will be employed. Quantitative demographic variables among the three groups will be compared using either one-way ANOVA or Kruskal-Wallis tests, depending on the data distribution. The chi-squared test will be used to compare categorical variables between the groups.
If there are no significant differences in outcome measures among the three groups at baseline, a 3 × 3 repeated measures analysis of variance will be conducted. Group (occupation-based interventions with motivational strategy; occupation-based interventions without motivational strategy; conventional interventions) will be the between-subject factor, and time (baseline, session 10, session 18, post-intervention, follow-up) will be the within-subject factor. Multiple comparisons will be performed using the Tukey post hoc test. However, if significant differences are observed in outcome measures among the three groups at baseline, a separate regression analysis will be conducted. Group and baseline will be considered as covariates at each assessment point [i.e., session 10, session 18, post-intervention (week 9), follow-up (week 25)]. Differences will be calculated using the Kruskal-Wallis test. All statistical analyses will consider a significance level of P < 0.05 (two-tailed). The data will be entered and analyzed using SPSS version 13.
4. Discussion
The forthcoming trial aims to be groundbreak and pioneering, introducing a fresh perspective may lay the groundwork for future research. While previous studies (47, 48) have explored the impact of personalized OT, certain methodological challenges, such as the intensity and structure of the intervention, have hindered the ability to draw sturdy conclusions. Furthermore, a recent systematic review has indicated that these individualized OT interventions offer a moderate level of evidence for enhancing abilities in performing and participating in activities of daily living (ADL) (49). Moreover, there is a lack of research exploring the use of activities in interventions based on OT concepts and utilizing models from this discipline. In response to these concerns, this trial will adopt a structured, motivation-based, and occupation-focused approach. The intervention will be administered in an activity-based form and method. Consequently, future studies can compare the effectiveness of this intervention with other exercise-based interventions, while also considering the importance of a motivated occupational therapist to provide a holistic approach, aligned with the core principles of OT (50).
The anticipated findings from this study are expected to enrich the knowledge of clinicians, empowering them to make evidence-based and comprehensive decisions. Additionally, this protocol based on activities will help occupational therapists structure a diverse range of intervention sessions. By implementing a protocol that aligns with the concept of the MOHO, therapists can effectively motivate their patients, leading to enhanced outcomes in clinical interventions. However, it is important to acknowledge the limitations of this research. First, the delivery of the intervention may face disruptions due to factors like inclement weather or the unavailability of therapists and patients due to illness. To mitigate these challenges, the team will incorporate additional therapists to cover any sick leaves. Second, there is a possibility that patients may underestimate or over-report the trial's outcomes in self-report measures. Other limitations of this study include the reliance solely on a motion analysis system without incorporating EMG and MRI, potentially leading to less detailed outcomes. Furthermore, only four additional activities identified by the COPM will be included in the study.
In conclusion, the outcomes of this proposed trial have the potential to greatly enhance participation, which is the ultimate goal for both patients and therapists. This improvement in participation may also extend to other motor and non-motor symptoms experienced by individuals living with PD.
4.1. Key Messages
• Activity-based interventions, developed according to upper limb taxonomy and the Gentile theory of motor learning, in collaboration with an expert panel, aim to promote participation in patients with PD.
• Motivational interventions, designed based on the motivational strategies of the MOHO and input from an expert panel, aim to enhance participation in patients with PD.
• Evaluation of kinematic parameters of symmetric and asymmetric bimanual function and coordination post-intervention aims to provide detailed information about the motor function of the upper extremity.
4.2. Trial Status
The development of activity-based and motivational strategy concepts has been finalized. Recruitment commenced in November 2019, with assessments for remaining participants expected to conclude by July 2020. Any necessary alterations to the protocol will be submitted for approval to the Iranian Registry of Clinical Trials. The current protocol (code: IRCT 20140304016830N12) is version 1.0, dated May 27, 2020.