1. Background
The term "sleep inertia" denotes a transient phase of drowsiness, confusion, and reduced cognitive abilities experienced immediately after waking up, as opposed to the state before falling asleep (1). Research highlights that sleep inertia affects a range of functions, including motor skills, cognitive performance (2), and alertness, with complex tasks being more vulnerable than simpler ones (3, 4). While vocal function is essential for communication and self-confidence (5), few studies have examined how sleep inertia impacts voice quality. Legros et al. (6) observed significant changes in fundamental frequency following short naps, and further research confirmed that sleep inertia also affects vowel sounds (7). Cavallero and Versace (8) reported that sleep deprivation exacerbates the effects of sleep inertia. Vocal fatigue, a result of sleep inertia, can lead to significant changes in voice quality (9, 10). Sleep inertia appears to be closely tied to the duration of slow-wave sleep, being more pronounced after longer naps with increased slow-wave activity. Additionally, voice disorders are influenced by sleep quality and stress (11, 12). While its direct impact on voice production requires further investigation, sleep inertia may affect muscle control, breathing, and resonance. Additionally, natural physiological processes during nocturnal sleep lead to a deeper, hoarser voice (13) upon waking, potentially impairing vocal performance for up to 30 minutes (14, 15).
The impact of sleep inertia on voice quality remains unclear, with mixed findings primarily focused on the effects following short-term naps during the day rather than after a full night's sleep (6, 7, 16). Icht et al. (17) recorded voice samples both after a night's sleep and after 24 hours of sleep deprivation, but comparing the voice before and immediately after sleep is essential due to the nature of sleep inertia. Previous research (6, 17) has mainly examined the acoustic qualities of voice. Despite advanced tools for acoustic, aerodynamic, and vocal imaging, auditory-perceptual evaluation is still the preferred method among voice therapists (18, 19). Our study focuses on key acoustic voice parameters such as jitter and shimmer, which assess the stability and smoothness of voice, with higher values often indicating roughness or breathiness and suggesting potential vocal issues. Monitoring these parameters can aid in assessing and enhancing overall voice quality (20). Another critical parameter, the harmonics-to-noise ratio (HNR), quantifies the level of additive noise in the voice signal (21). Additionally, smoothed cepstral peak prominence (CPPS) is recognized as a reliable metric for assessing dysphonic voice qualities in both single vowel and connected speech tasks (22, 23). Based on previous studies, a series of vocal tasks were selected for analysis: Sustained phonation, spontaneous speech, standard text reading, and maximum phonation time (MPT). These tasks offer a good variety and are ideal for analysis. Sustained phonation analyzes steady-state vocal qualities (24), spontaneous speech captures natural variations (25), standard text reading ensures uniformity, and MPT measures vocal fold endurance (26). This diverse selection aims to provide a comprehensive and accurate analysis of voice function. Recordings were made in a quiet room using a Zoom H6 recorder at a 44.1 kHz rate.
This study aimed to explore the impact of sleep inertia on acoustic and perceptual voice parameters in young adults by recording voices before and immediately after night sleep in both genders and comparing the outcomes. Unlike prior studies, this research recorded voices both before and after sleep to evaluate a wider range of acoustic parameters. It utilized the GRBAS Scale, recommended by Icht et al. (17) for comprehensive perceptual voice assessment, covering Grade, Roughness, Breathiness, Asthenia, and Strain from 0 (normal) to 3 (severe). Two experienced therapists conducted blind assessments using earphones, reviewing all samples except MPT. The study sought to understand how sleep inertia affected morning voice clarity, which is crucial in professions requiring optimal vocal performance upon waking. The research also examined how voice quality varied post-sleep between males and females, providing insights into the specific effects of sleep inertia on voice characteristics.
2. Methods
2.1. Participants
The study was confirmed by the Ethical Committee of Hamadan University of Medical Sciences ( IR.UMSHA.REC.1401.974) and comprised 90 healthy university students (native Persian speakers) with no reported voice issues affecting pitch, volume, tone, or overall quality. This group included 45 females (average age: 21.86 years, range: 19 - 24) and 45 males (average age: 23.75 years, range: 18 - 30). Participants were selected via convenience sampling, meeting inclusion criteria. The criteria for inclusion encompassed no history of head and neck surgery, absence of current voice disorders, normal hearing, no ongoing cold symptoms, and no voice changes related to smoking. Additionally, the demographic form recorded participants' consumption of liquids such as water and caffeinated drinks. To prevent potential confounding effects, participants were instructed not to consume any liquids after waking up during the second recording session.
2.2. Procedure
During data collection, two trained speech-language pathology students recorded participants' voices and provided thorough briefings on the study's objectives and protocols. Each participant's voice was recorded under two specific conditions, timed and sequenced to strategically analyze the effects of sleep on vocal characteristics. The sequence of recordings was structured as follows:
Initial recording: Conducted at night just before the participant went to sleep, this session aimed to document the baseline vocal condition following a typical day, unaffected by sleep.
Second recording: Scheduled for the morning immediately after the participant awoke, this session was designed to assess any changes in the voice that might have occurred due to sleep, comparing it with the baseline from the previous night.
Participants were told to return the next morning for a second recording after the initial night session. They self-reported their night's sleep duration along with their age and Body Mass Index (BMI) in the demographic questionnaire. Body Mass Index is calculated by dividing weight in kilograms by height in meters squared (kg/m2) and classifies individuals as underweight, normal, overweight, or obese. Participants' sleep quality or sleep disorders were documented in their demographic information using the "Pittsburgh Sleep Quality Index" (PSQI) Questionnaire. The PSQI is a 19-item self-report questionnaire used to assess sleep quality. It is one of the most widely used and important tools for evaluating sleep quality.
2.3. Voice Sampling
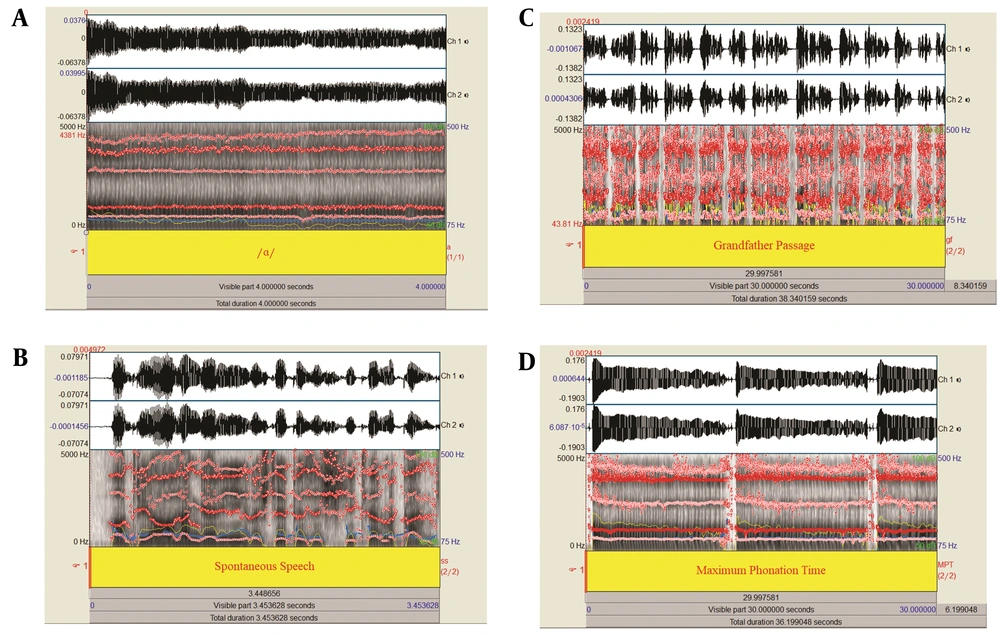
Previous research has shown that sustained vowels and connected speech are ideal for recording voice samples (24). One of the voice recordings used in the study was a standard text reading task ensuring uniform speech samples across individuals, minimizing the influence of speech speed and patterns. Despite this, spontaneous speech was included because it effectively highlights differences between normal and abnormal voice production (25). Maximum Phonation Time, a common clinical measure, assesses the phonation mechanism by determining the longest duration a vowel like /a/ can be sustained (26). All of the voice recordings employed for acoustic analysis included sustained phonation, spontaneous speech, standard text reading, and MPT, as illustrated in Figure 1. Given the variations in voice acoustic parameters noted in earlier studies for different speech tasks (27), our study incorporates diverse voice samples for comprehensive acoustic analysis.
During each experimental session, participants were required to produce four types of voice samples in both recording sessions:
(1) Sustained phonation: Participants sustained the vowel /a/ for five seconds at a comfortable loudness and constant pitch.
(2) Spontaneous speech: Participants introduced themselves by stating their first and last names, field of study, and academic semester.
(3) Standard text reading: Participants read the Persian version of "The Grandfather Passage" (25).
(4) Maximum phonation time: Participants took a deep breath and then sustained the vowel /a/ for as long as possible, repeating this three times.
All voice samples were recorded in a quiet room in the student dormitory of Hamadan University of Medical Sciences. A Zoom H6 handy recorder (Zoom Corporation, Japan) was used, set to a sampling rate of 44.1 kHz. The Zoom H6 is a portable recorder featuring interchangeable mic capsules, six-track simultaneous recording up to 24-bit/96kHz, and four XLR/TRS inputs. It doubles as a multi-channel audio interface, making it ideal for versatile audio recording needs. The recorder was placed horizontally, slightly above the participant's lips at an equal distance of 10 cm from their mouth to record their voices.
2.4. Acoustic Analysis and Maximum Phonation Time
Voice samples were analyzed using Praat software version 6.2.03 (28). For sustained phonation tasks, analysis focused on a 4-second middle segment, excluding the initial and final half-seconds (29). All voice samples, except for MPT, were assessed for fundamental frequency (F0) in Hz, standard deviation of F0 (SD F0) in Hz, voice intensity in dB, HNR in dB, jitter, shimmer in percentage, and cepstral peak prominence-smoothed (CPPS) in dB. Additionally, the first two formant frequencies (F1, F2) of the vowel /a/ were also measured. Maximum phonation time was calculated as the average of three attempts per participant. The CPPS calculation followed the method described by Watts et al. (30). Each voice sample type (sustained phonation /a/, spontaneous speech, grandfather passage reading, and MPT) was analyzed separately for each participant in a blind manner to avoid bias.
2.5. Auditory-Perceptual Evaluation
The GRBAS Scale was used for auditory-perceptual evaluations of voice samples, assessing Grade (G), Roughness (R), Breathiness (B), Asthenia (A), and Strain (S) on a scale from zero (normal) to three (severe problem). Two experienced voice therapists with over four years of practice performed blind assessments in a quiet room using bilateral earphones, reviewing all samples except for MPT. To ensure unbiased evaluations, voice samples were randomly coded, keeping raters unaware of the subjects' identities and the recording times. Inter-rater reliability was measured by having the therapists independently score the samples twice, one week apart. The GRBAS Scale was used for conducting auditory-perceptual evaluations of the recorded voice samples. The inter-rater reliability for the GRBAS parameters was measured using the weighted kappa coefficient, showing strong agreement: Grade (0.751), roughness (0.807), breathiness (0.839), asthenia (0.779), and strain (0.760), all considered excellent as values above 0.75. Intra-rater reliability was also assessed by having therapists reevaluate 15% of the samples, resulting in consistent kappa values: Grade (0.845), roughness (0.846), breathiness (0.876), asthenia (0.828), and strain (0.714), indicating high agreement for most parameters.
2.6. Statistical Analysis
Statistical analysis was conducted using SPSS-19 software (SPSS Inc., Chicago, Illinois). A Shapiro-Wilk test was used to assess the normality of the differences between two dependent variables in both acoustic and auditory-perceptual analyses. A paired t-test was then conducted to compare these variables. Due to gender differences in voice characteristics and parameters, all analyses were performed separately for male and female participants at a significance level of 0.05.
3. Results
3.1. Demographic Characteristics
Demographic characteristics, including age, BMI, and sleep duration (in minutes), are depicted in Table 1. Applying the independent samples t-test showed a significant difference in sleep duration between the two gender groups (P = 0.015). Females reported an average sleep duration of 43.2 minutes longer than males.
| Variables | Age | BMI | Sleep Duration |
|---|---|---|---|
| Sex | |||
| Female | 21.86 (1.23) | 22.40 (3.30) | 406.8 (79.2) |
| Male | 23.75 (2.89) | 23.73 (3.55) | 363.6 (112.8) |
| P-value b | < 0.001 | 0.804 | < 0.05 |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as mean ± SD.
b Independent samples t-test, statistically significant difference (P < 0.05).
3.2. Acoustic Analysis of Voice Quality, Maximum Phonation Time, and Auditory-Perceptual Evaluation
The mean and standard deviation for various acoustic voice parameters across three voice samples (sustained phonation /a/, spontaneous speech, and grandfather passage), measured before and after sleep in both male and female participants, along with the effect size values and their related rankings (low, medium, and high effect size) for each acoustic parameter, are presented in Table 2.
| Type of Voice Sample and Acoustic Parameter | Before Nocturnal Sleep | After Nocturnal Sleep | Difference (After-Before) | Effect Size (Rankings: H, M, L) | 95% CI for Difference | P- Value b |
|---|---|---|---|---|---|---|
| (a) Female participants, Sustained phonation /a/ | ||||||
| Mean F0 (Hz) | 215.40 (21.12) | 197.36 (35.69) | - 18.04 (29.74) | - 0.60 (M) | (- 27.29, - 8.79) | < 0.001 |
| SD1 F0 (Hz) | 5.25 (11.29) | 19.30 (19.30) | 14.05 (21.81) | 0.64 (M) | (6.43, 21.67) | < 0.001 |
| Mean intensity (dB) | 58.94 (4.49) | 52.90 (3.33) | - 6.04 (4.67) | - 1.29 (H) | (- 7.12, - 4.96) | < 0.001 |
| HNR (dB) | 21.86 (3.31) | 17.57 (3.85) | - 4.28 (3.44) | - 1.24 (H) | (- 5.09, - 3.47) | < 0.001 |
| CPPS (dB) | 13.13 (1.40) | 10.28 (1.91) | - 2.85 (2.28) | - 1.25 (H) | (- 3.37, - 2.33) | < 0.001 |
| Jitter | 0.39 (0.24) | 0.80 (0.50) | 0.31 (0.45) | 0.68 (M) | (0.19, 0.43) | < 0.001 |
| Shimmer | 3.25 (1.24) | 5.43 (2.19) | 1.34 (2.13) | 0.62 (M) | (0.77, 1.91) | < 0.001 |
| Mean F1 (Hz) | 691.45 (83.52) | 553.68 (112.70) | - 137.76 (93.11) | - 1.47 (H) | (- 171.92, - 103.60) | < 0.001 |
| Mean F2 (Hz) | 1202.78 (86.89) | 1159.18 (79.44) | - 43.59 (103.15) | - 0.42 (M) | (- 75.52, - 11.66) | < 0.001 |
| Spontaneous speech | ||||||
| Mean F0 (Hz) | 220.87 (21.40) | 203.10 (31.54) | - 17.77 (27.88) | - 0.63 (M) | (- 27.74, - 7.80) | < 0.001 |
| SD1 F0 (Hz) | 44.92 (23.08) | 52.27 (24.75) | 7.35 (21.51) | 0.34 (L) | (0.50, 14.20) | < 0.05 |
| Mean intensity (dB) | 56.20 (3.23) | 51.02 (2.96) | - 5.17 (2.85) | - 1.81 (H) | (- 6.33, - 4.01) | < 0.001 |
| HNR (dB) | 16.19 (1.52) | 14.90 (2.19) | - 1.28 (2.21) | - 0.57 (M) | (- 2.14, - 0.42) | < 0.001 |
| CPPS (dB) | 8.95 (1.23) | 7.05 (1.22) | - 1.90 (1.450) | - 1.31 (H) | (- 2.33, - 1.47) | < 0.001 |
| Jitter | 1.55 (0.30) | 1.87 (0.50) | 0.31 (0.45) | 0.68 (M) | (0.14, 0.48) | < 0.001 |
| Shimmer | 6.71 (1.01) | 8.05 (2.07) | 1.34 (2.13) | 0.62 (M) | (0.52, 2.16) | < 0.001 |
| Grandfather passage | ||||||
| Mean F0 (Hz) | 210.82 (18.31) | 194.95 (23.57) | - 15.87 (20.82) | - 0.76 (H) | (- 23.32, - 8.42) | < 0.001 |
| SD1 F0 (Hz) | 45.15 (10.88) | 57.55 (14.00) | 12.39 (13.06) | 0.94 (H) | (7.82, 16.96) | < 0.001 |
| Mean intensity (dB) | 57.26 (3.26) | 52.36 (3.05) | - 4.89 (2.90) | 1.68 (H) | (- 5.90, - 3.88) | < 0.001 |
| HNR (dB) | 15.60 (1.23) | 14.54 (1.96) | - 1.06 (1.57) | - 0.67 (M) | (- 1.59, - 0.53) | < 0.001 |
| CPPS (dB) | 9.02 (0.94) | 7.24 (1.15) | - 1.78 (1.16) | - 1.53 (H) | (- 2.24, - 1.32) | < 0.001 |
| Jitter | 1.66 (0.27) | 2.04 (0.50) | 0.37 (0.42) | 0.88 (H) | (0.20, 0.54) | < 0.001 |
| Shimmer | 7.25 (0.73) | 8.32 (1.67) | 1.07 (1.60) | 0.66 (M) | (0.51, 1.63) | < 0.001 |
| Maximum phonation time (MPT) | ||||||
| MPT (s) | 13.87 (4.28) | 11.40 (3.48) | - 2.47 (2.95) | - 0.83 (H) | (- 3.53, - 1.41) | < 0.001 |
| (b) Male participants sustained phonation /a/ | ||||||
| Mean F0 (Hz) | 117.24 (14.29) | 106.56 (12.00) | - 10.67 (9.53) | - 1.11 (H) | (- 14.08, - 7.26) | < 0.001 |
| SD1 F0 (Hz) | 1.28 (0.67) | 1.76 (1.39) | 0.48 (1.41) | 0.34 (L) | (- 0.03, 0.99) | < 0.001 |
| Mean intensity (dB) | 61.83 (3.50) | 59.20 (4.27) | - 2.62 (3.87) | - 0.67 (M) | (- 4.01, - 1.23) | < 0.001 |
| HNR (dB) | 21.31 (3.82) | 19.43 (3.95) | - 1.87 (3.23) | - 0.57 (M) | (- 3.02, - 0.72) | < 0.001 |
| CPPS (dB) | 15.87 (2.48) | 14.18 (2.83) | - 1.69 (2.50) | - 0.67 (M) | (- 2.59, - 0.79) | < 0.001 |
| Jitter | 0.42 (0.25) | 0.93 (1.40) | 0.50 (1.41) | 0.35 (L) | (- 0.01, 1.01) | < 0.05 |
| Shimmer | 3.54 (1.80) | 4.24 (2.65) | 0.69 (2.36) | 0.29 (L) | (- 0.15, 1.53) | 0.054 |
| Mean F1 (Hz) | 566.76 (47.71) | 530.80 (63.46) | - 35.95 (41.44) | - 0.86 (H) | (- 50.78, - 21.12) | < 0.001 |
| Mean F2 (Hz) | 1031.18 (71.54) | 1019.62 (72.59) | - 11.55 (75.26) | - 0.15 (L) | (- 38.47, 15.37) | 0.309 |
| Spontaneous speech | ||||||
| Mean F0 (Hz) | 125.74 (15.04) | 115.72 (13.35) | - 10.02 (12.58) | - 0.79 (H) | (- 14.53, - 5.51) | < 0.001 |
| SD1 F0 (Hz) | 33.53 (28.27) | 33.97 (35.68) | 0.43 (41.20) | 0.01 (L) | (- 14.31, 15.17) | 0.943 |
| Mean intensity (dB) | 57.50 (2.28) | 55.80 (2.66) | - 1.69 (2.69) | - 0.62 (M) | (- 2.65, - 0.73) | < 0.001 |
| HNR (dB) | 12.97 (2.26) | 12.62 (2.14) | - 0.35 (1.78) | - 0.19 (L) | (- 1.00, 0.30) | 0.190 |
| CPPS (dB) | 8.74 (1.36) | 8.06 (1.32) | - 0.68 (1.23) | - 0.55 (M) | (- 1.11, - 0.25) | < 0.001 |
| Jitter | 2.16 (0.56) | 2.65 (1.07) | 0.48 (1.16) | 0.41 (M) | (0.07, 0.89) | < 0.001 |
| Shimmer | 9.79 (2.39) | 10.31 (2.17) | 0.51 (2.18) | 0.23 (L) | (- 0.28, 1.30) | 0.119 |
| Grandfather passage | ||||||
| Mean F0 (Hz) | 125.02 (15.16) | 113.63 (12.60) | - 11.38 (9.61) | - 1.18 (H) | (- 14.81, - 7.95) | < 0.001 |
| SD1 F0 (Hz) | 31.28 (15.34) | 35.78 (22.32) | 4.50 (16.15) | 0.27 (L) | (- 1.28, 10.28) | < 0.001 |
| Mean intensity (dB) | 59.61 (2.76) | 56.78 (2.59) | - 2.83 (2.31) | - 1.22 (H) | (- 3.65, - 2.01) | < 0.001 |
| HNR (dB) | 11.77 (1.83) | 11.87 (1.99) | 0.10 (1.27) | 0.07 (L) | (- 0.35, 0.55) | 0.597 |
| CPPS (dB) | 8.58 (1.04) | 8.20 (1.18) | - 0.37 (1.00) | - 0.37 (L) | (- 0.72, - 0.02) | < 0.05 |
| Jitter | 2.26 (0.35) | 2.63 (0.67) | 0.37 (0.58) | 0.63 (M) | (0.15, 0.59) | < 0.001 |
| Shimmer | 10.53 (1.76) | 10.70 (2.04) | 0.17 (1.66) | 0.10 (L) | (- 0.42, 0.76) | 0.488 |
| Maximum phonation time | ||||||
| MPT (s) | 20.19 (6.55) | 18.28 (6.41) | - 1.91 (5.56) | - 0.34 (L) | (- 3.89, - 0.93) | < 0.05 |
Abbreviations: MPT, maximum phonation time; SD1 F0 (Hz), Standard deviation F0 (Hz); CI, confidence interval; H, high effect size; M, medium effect size; L, low effect size.
a Values are expressed as No. (%) or mean ± SD.
b Paired t-test, statistically significant difference (P < 0.05).
Significant findings include a decrease in mean F0, mean intensity, HNR, and CPPS for all voice samples in females, and specifically F1 and F2 during sustained phonation /a/. Additionally, increases in the SD F0, jitter, and shimmer were observed across all samples. In males, there were notable decreases in mean F0, mean intensity, and CPPS across all voice samples, while jitter showed a significant increase across all samples. Both genders also experienced a significant reduction in MPT. The effect size results showed that in the female group, all acoustic parameters exhibited medium to high effect sizes, except for the SD F0 in spontaneous speech, which was low. Conversely, in the male group, the effect sizes for the studied acoustic parameters ranged from low to high.
Table 2 provides detailed statistics and effect sizes for each parameter, reflecting the impact of sleep inertia on vocal characteristics and highlighting gender differences in these effects.
3.3. Acoustic Analysis of Voice Quality, Maximum Phonation Time, and Auditory-Perceptual Evaluation
- The mean and standard deviation for various acoustic voice parameters across three voice samples (sustained phonation /a/, spontaneous speech, and grandfather passage), measured before and after sleep in both male and female participants, along with the effect size values and their related rankings (low, medium, and high effect size) for each acoustic parameter, are presented in Table 2.
- Significant findings include a decrease in mean F0, mean intensity, HNR, and CPPS for all voice samples in females, and specifically F1 and F2 during sustained phonation /a/. Additionally, increases in the SD F0, jitter, and shimmer were observed across all samples. In males, there were notable decreases in mean F0, mean intensity, and CPPS across all voice samples, while jitter showed a significant increase across all samples. Both genders also experienced a significant reduction in MPT. The effect size results showed that in the female group, all acoustic parameters exhibited medium to high effect sizes, except for the SD F0 in spontaneous speech, which was low. Conversely, in the male group, the effect sizes for the studied acoustic parameters ranged from low to high.
Table 2 provides detailed statistics and effect sizes for each parameter, reflecting the impact of sleep inertia on vocal characteristics and highlighting gender differences in these effects.
3.4. Auditory-Perceptual Analysis
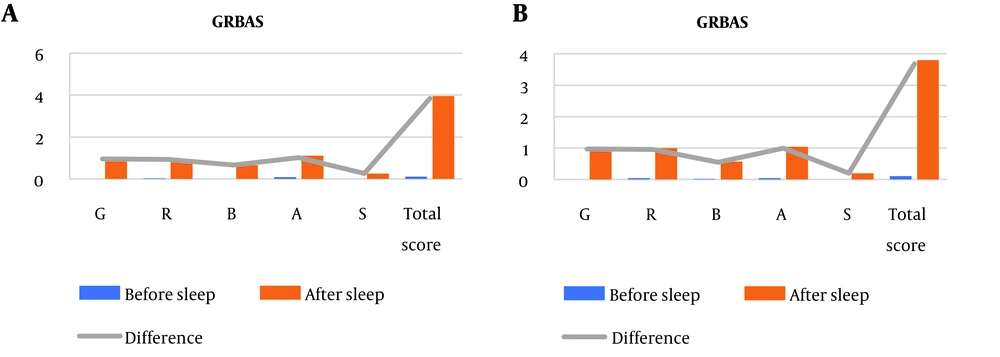
- The results of auditory-perceptual analysis by gender, (a) for females and (b) for males, are shown in Table 3. For a better review of the results, the GRBAS test findings in female and male groups are presented in Figure 2. The difference in the total GRBAS score was 3.84 (P < 0.001) for females and 3.58 (P < 0.001) for males, indicating slightly greater auditory-perceptual changes in females compared to males. The effect size for all GRBAS subscales was high for both genders, with the exception of strain, which exhibited a medium effect size.
Figure 2 shows the changes in mean values of GRBAS auditory-perceptual parameters before and after night sleep, illustrating the overall impact of sleep inertia on voice quality for both genders.
| GRBAS Scale Items | Before Nocturnal Sleep | After Nocturnal Sleep | Difference (After-Before) | Effect Size (Rankings: H, M, L) | 95% CI for Difference | P-Value b |
|---|---|---|---|---|---|---|
| (a) Females | ||||||
| Grade | 0.00 (0.00) | 0.95 (0.79) | 0.95 (0.79) | 1.20 (H) | (0.75, 1.15) | < 0.001 |
| Roughness | 0.02 (0.14) | 0.95 (0.52) | 0.93 (0.53) | 1.75 (H) | (0.78, 1.08) | < 0.001 |
| Breathiness | 0.00 (0.00) | 0.66 (0.63) | 0.66 (0.63) | 1.04 (H) | (0.49, 0.83) | < 0.001 |
| Asthenia | 0.08 (0.28) | 1.11 (0.53) | 1.02 (0.62) | 1.64 (H) | (0.86, 1.18) | < 0.001 |
| Strain | 0.00 (0.00) | 0.26 (0.44) | 0.26 (0.44) | 0.59 (M) | (0.14, 0.38) | < 0.001 |
| Total score | 0.11 (0.38) | 3.95 (2.23) | 3.84 (2.26) | 1.69 (H) | (3.28, 4.40) | < 0.001 |
| (b) Males | ||||||
| Grade | 0.00 (0.00) | 0.97 (0.75) | 0.97 (0.75) | 1.29 (H) | (0.77, 1.17) | < 0.001 |
| Roughness | 0.04 (0.20) | 1.00 (0.52) | 0.96 (0.52) | 1.84 (H) | (0.82, 1.10) | < 0.001 |
| Breathiness | 0.02 (0.14) | 0.57 (0.54) | 0.55 (0.54) | 1.01 (H) | (0.42, 0.68) | < 0.001 |
| Asthenia | 0.04 (0.20) | 1.04 (0.42) | 1.00 (0.47) | 2.12 (H) | (0.86, 1.14) | < 0.001 |
| Strain | 0.00 (0.00) | 0.20 (0.40) | 0.20 (0.40) | 0.5 (M) | (0.10, 0.30) | < 0.001 |
| Total score | 0.11 (0.31) | 3.80 (2.00) | 3.68 (1.92) | 1.91 (H) | (3.12, 4.24) | < 0.001 |
Abbreviations: MPT, maximum phonation time; SD1 F0 (Hz), standard deviation F0 (Hz); CI, confidence interval; H, high effect size; M, medium effect size; L, low effect size.
a Values are expressed as No. (%) or mean ± SD.
b Paired t-test, statistically significant difference (P < 0.05).
4. Discussion
"Sleep inertia" refers to drowsiness and reduced cognitive function upon waking. While its effects on motor skills and cognition are well-studied (6, 17, 31), its impact on voice quality remains less explored. This study fills this gap by analyzing voice changes before and after sleep using acoustic and auditory perceptual analyses.
This study noted significant reductions in mean F0 and mean intensity in both genders after night sleep, consistent with findings by Icht et al. (17), who studied 24-hour sleep deprivation. Contrarily, Legros et al. (6) reported increases in mean F0 after short naps, a discrepancy likely explained by different sleep durations between the studies.
Both males and females showed decreases in HNR and CPPS values post-sleep, in line with Icht et al. (17), who also observed a decrease in HNR after night sleep, with the reduction being more pronounced in women compared to men. The effect size of the reduction in HNR and CPPS in the female group compared to men ranged from medium to high, while it ranged from low to medium across different speech tasks. This suggests a noisier and more irregular voice after night sleep in women. Previous studies have not investigated the Effect Size of the differences in acoustic parameters after night sleep, making it impossible to compare this finding with past studies. Lower HNR values often seen in pathological voices (32). The CPP quantifies energy in the main harmonic, reflecting voice organization, while CPPS enhances this by averaging across quefrency and time domains for improved clarity (33, 34). Lower CPPS values are typically linked to pathological conditions, indicating more abnormal voices (33, 35). Our findings of reduced CPPS align with those of Hasanvand et al. (35), who observed similar trends in individuals with voice disorders.
Our study observed increased jitter and shimmer values for both sexes after sleep, consistent with previous findings (17). Standard deviation F0 values also rose significantly across most vocal tasks, except in spontaneous speech. Jitter, shimmer, and SD F0, which measure short-term frequency and amplitude variations, are crucial for detecting voice abnormalities and assessing the severity of voice deviations (36-38). Higher values of jitter and shimmer, along with variable SD F0, indicate disturbances in voice quality, suggesting that sleep affects voice consistency and quality (39, 40).
After night sleep, both men and women showed a significant decrease in MPT, an essential tool for assessing voice disorders and treatment efficacy (41). Decreases in MPT can indicate issues like insufficient inspiratory volume or glottal resistance, leading to breathiness (42). Consistent with earlier studies (43, 44), MPT is lower for patients with voice disorders than normal subjects. In this study, pre-sleep MPT values were normal—13.87 seconds for women and 20.19 for men—but post-sleep, MPT decreased for both genders, particularly falling below normal for women (45). This reduction is likely due to overnight relaxation of respiratory muscles, weakening muscle strength and affecting respiratory support, as described by Schwarz et al. (46). These results underscore the importance of further research into post-sleep changes in MPT and respiratory functions, advocating for more detailed aerodynamic assessments.
Perceptual analysis with the GRBAS scale revealed significant increases in all perceptual parameters for both genders, suggesting that voice quality becomes harsher, rougher, breathier, weaker, and more strained after sleep due to the effects of sleep inertia. The effect sizes for differences in all GRBAS subscales for both genders after night sleep were high, except for "strain," which was medium. This may be attributed to the inherent characteristics of the "strain" subscale.
The statistical significance of the reported changes in acoustic parameters and GRBAS scores reveals important insights into the impact of sleep inertia on voice quality in both male and female participants. Females showed significant decreases in F0, intensity, HNR, and CPPS across all voice samples, with increased SD F0, jitter, and shimmer. Males experienced reductions in F0, intensity, CPPS, HNR, and F1 during sustained phonation /a/, along with increased SD F0 and jitter. Both genders also saw reduced MPT, indicating compromised vocal endurance. Effect size analysis revealed medium to high effects in females for most parameters, whereas males exhibited varied effects. Auditory-perceptual analysis using the GRBAS scale showed significantly higher total score changes in females (3.84, P < 0.001) compared to males (3.58, P < 0.001), with high effect sizes across most GRBAS subscales except for strain, which had a medium effect size. The more pronounced acoustic and perceptual changes in women's voices compared to men's can be attributed to gender-related anatomical and physiological differences in the vocal folds (47). Female vocal folds are approximately 20 - 30% thinner and contain less collagen, elastin, and hyaluronic acid (48, 49), making them more susceptible to injury and more sensitive to physiological changes during sleep that affect voice quality (50). Hormonal fluctuations, especially related to the menstrual cycle, further influence vocal fold function and voice quality in women. Additionally, effect size comparisons for most acoustic parameters and MPT revealed that women's voices are more affected by nocturnal sleep than men's. Consequently, all acoustic parameters, including fundamental frequency, jitter, shimmer, and harmonic-to-noise ratio (HNR), show significant changes upon waking in females, whereas in males, only certain parameters like shimmer and HNR are significantly affected. Maximum phonation time is also more impacted in women. These findings underscore the importance of tailored voice therapy, particularly for female patients, and highlight the need for awareness and management of sleep patterns and wake-up routines among professionals who rely heavily on their voices.
In general, the possible reasons may explain the observed changes in voice parameters summarized as follows:
Increased muscle relaxation and reduced vocal fold activity: During sleep, muscle relaxation leads to partial larynx closure, disrupting vocal fold vibrations and reducing voice quality, resulting in lower mean F0, mean intensity, and MPT, and higher jitter, shimmer, and SD F0 (31). Relaxation also shifts the positions of the tongue, palate, and vocal tract, affecting formant frequencies (51). Tongue positioning significantly influences formant frequencies, crucial for determining vowel sounds. F1 is inversely correlated with tongue height (higher F1, lower tongue), and F2 is associated with tongue backness and lip rounding (higher F2, more front tongue, less rounded lips) (52). Upon waking, reduced muscle tension alters vocal tract resonances, changing mean F1 and F2, suggesting a more elevated and posterior tongue position during sleep (53). Future studies should consider using advanced imaging technologies like MRI or ultrasound to directly examine tongue positioning during sleep and its effects on vocal tract configurations and voice production.
Decreased throat lubrication and vocal fold hydration: Sleep reduces saliva production and swallowing, leading to fluid buildup and mucus on the vocal folds, which can cause slight swelling (27). This swelling disrupts vocal fold vibrations, resulting in a rougher voice after sleep (13). Additionally, mouth breathing during sleep dehydrates the airway and dries vocal fold membranes (54), potentially causing vocal strain (13, 55). These conditions can lead to decreased CPPS and HNR values, and increased jitter, shimmer, and SD F0 values in acoustic assessments, along with elevated perceptual parameters post-sleep.
Nocturnal acid reflux: Nocturnal acid reflux is associated with the development of laryngopharyngeal reflux disease (LPRD), which is frequently observed in individuals with vocal disorders. In fact, LPRD is diagnosed in over 50% of patients who present with voice-related complaints (56). Acid reflux can also lead to a hoarse voice in the morning (57). Patients with laryngopharyngeal reflux usually complain of voice problems such as burning in the throat area, throat clearing, chronic cough, hoarseness, or sore throat, especially in the morning (15, 58).
4.1. Limitations
The current study has some limitations. Firstly, it targets a young demographic (18 - 30 years) and may not represent voice changes across all age groups, limiting its generalizability. Future research should include a broader age range, including older and younger individuals, in order to enhance the analysis of voice quality. Secondly, the study did not consider confounding factors such as diet, which could influence the outcomes. Future studies should explore these variables to better understand sleep's impact on voice quality. Lastly, the study lacked a third measurement to assess voice normalization post-sleep disturbance. Including a follow-up measurement in future studies is recommended to monitor the return to normal voice states. Furthermore, in addition to the previously mentioned points, utilizing advanced technologies like ultrasound imaging of the tongue can provide a more precise assessment of tongue movements and positioning during sleep. Additionally, future research should explore the intricate connection between various sleep stages and their impact on voice production.
4.2. Conclusions
This study demonstrates that sleep inertia, characterized by reduced functionality and alertness upon waking, negatively impacts voice performance in university students aged 18 to 30, leading to decreased voice quality in both genders. By focusing on this specific age group, the research highlights the significant effects of sleep inertia on young adult voice quality, emphasizing the need for further investigation in this area. Notably, the study's strengths include its inclusion of both genders, a substantial sample size, the use of the GRBAS scale for perceptual evaluation, and a comprehensive analysis of sleep inertia.
These findings enhance our understanding of the relationship between sleep and voice health, which is crucial for improving clinical assessments and treatments of voice disorders in young adults. Voice therapists should consider sleep patterns and inertia when planning sessions, especially for female students who may experience greater voice deterioration upon waking. Scheduling therapy sessions when patients are least affected by sleep inertia can optimize vocal performance and outcomes. Promoting good sleep hygiene practices, such as maintaining regular sleep schedules and creating a restful environment, is vital to reduce the effects of sleep inertia.
Professionals in this age group who rely heavily on their voices, such as university students, singers, teachers, and call center employees, should be informed about the impact of sleep inertia. Employers might consider these effects when arranging work shifts, particularly for female staff who may need more time to overcome sleep inertia. Additionally, this research can aid in developing recovery strategies and techniques for voice rehabilitation, helping speech and language pathologists improve voice treatments for young adults.