1. Context
Overactive bladder (OAB) is a symptomatic syndrome indicating lower urinary tract dysfunction, as defined by the International Continence Society (ICS) classification (1). According to this definition, OAB involves a sudden, urgent need to urinate—with or without incontinence—often characterized by increased frequency and nocturia, in the absence of a urinary tract infection (UTI) or other underlying pathologies. Patients presenting with these symptoms are diagnosed with either dry OAB (without incontinence) or wet OAB (with incontinence), with approximately 66% experiencing dry OAB and 33% wet OAB. Many individuals with OAB also suffer from painful bladder syndrome (PBS), also known as interstitial cystitis—a chronic condition marked by bladder pain and symptoms like urgency and frequency (2).
Overactive bladder syndrome has a prevalence ranging from 1.5% to 36.4%, significantly impacting patients' quality of life and imposing economic burdens on the healthcare system. Compared to healthy individuals, those with OAB are more likely to experience anxiety, depression, social isolation, reduced confidence, and impaired interpersonal relationships (3). Current treatments for OAB include behavioral therapy, pharmacotherapy, minimally invasive procedures, and other surgical options (4). Newer therapeutic methods have emerged, such as botulinum neurotoxin, sacral nerve neuromodulation (SNM), and posterior tibial nerve stimulation (PTNS) (5).
According to the European Association of Urology (EAU), American Urological Association (AUA), and National Institute for Health and Care Excellence (NICE), PTNS is recommended as a minimally invasive neuromodulation technique for OAB. Posterior tibial nerve stimulation received FDA approval in 2000 (6). Unlike acupuncture, PTNS targets an anatomical site rather than energy pathways (7) and is administered via two methods: P-PTNS and T-PTNS (3). In the percutaneous method, nerve stimulation is delivered through a needle electrode, while the transcutaneous method uses a surface electrode with a reference electrode placed on the same leg. Some studies have reported minor side effects, including pain, burning, bruising, and minor bleeding at the needle site post-treatment (3). The ease of use of PTNS allows patients to utilize it in home-based treatments.
Numerous studies have investigated the effects of PTNS on both painful bladder syndrome and overactive bladder syndrome (3, 8).
2. Objectives
This systematic review aimed to evaluate the effectiveness of posterior tibial nerve stimulation as a treatment for overactive bladder syndrome and to identify a potentially effective stimulation method.
3. Evidence Acquisition
The systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9). This systematic review was registered on PROSPERO (CRD42022367658). The following databases were searched: PubMed, Scopus, ProQuest, Google Scholar, Web of Science, and The Cochrane Library, without restrictions on time or language, up to April 2022.
3.1. Inclusion Criteria
The study included randomized controlled trials and nonrandomized quasi-experimental trials that met the inclusion criteria. The Patient, Problem/Intervention/Comparison/Outcome (PICO) criteria were as follows:
(1) Participants: Patients with idiopathic OAB or painful bladder symptoms who received PTNS as treatment, regardless of age, gender, or race.
(2) Interventions: Any type of posterior tibial nerve electrical stimulation, including P-PTNS or T-PTNS.
(3) Comparison: Control, sham, no treatment, routine treatment, medication, or placebo.
(4) Outcome: The primary outcome was the mean difference in voiding frequency, while secondary outcomes included mean voided volume, urinary incontinence episodes (UI), maximum cystometric capacity, urgency, and urgency urinary incontinence (UUI).
3.2. Exclusion Criteria
(1) Animal studies or studies using non-living samples
(2) Neurological OAB
(3) Full-text articles not available, even after contacting the authors
(4) Studies that did not specify treatment protocols in their articles or responses to inquiries
Electrical Stimulation Thresholds
The electrical stimulation threshold is defined at three levels:
(1) Sensory Threshold: The level of electrical stimulation where the person only feels the current.
(2) Motor threshold: The intensity of stimulation at which a motor response becomes apparent.
(3) Pain threshold: The intensity of stimulation surpasses the motor response limit and approaches the person’s pain and tolerance threshold.
Electrical Stimulation Methods
The electrical stimulation methods are categorized into six levels based on electrode placement:
- Method 1: The first electrode is positioned 3 - 5 cm above the medial malleolus, while the second electrode is placed around the medial malleolus.
- Method 2: The first electrode is positioned less than 3 cm above the medial malleolus, with the second electrode around the medial malleolus.
- Method 3: The first electrode is placed 3 - 5 cm above the medial malleolus, and the second electrode is positioned on the arch of the foot.
- Method 4: The first electrode is positioned less than 3 cm above the medial malleolus, with the second electrode on the arch of the foot.
- Method 5: The first electrode is positioned more than 5 cm above the medial malleolus, with the second electrode placed around the medial malleolus.
- Method 6: Both electrodes are placed on the tibial nerve on the foot at points not defined in the previous methods (Appendix 1 in Supplementary File).
3.3. Data Synthesis
The mean difference between the two groups was used as the effect index. Heterogeneity between studies was measured using the I-Squared (I²) Index, where an I² value greater than 50% indicated substantial heterogeneity. In cases of heterogeneity, the random-effects model was applied; otherwise, the fixed-effects model was used. A funnel plot was used to assess potential publication bias in the studies. A P-value of less than 0.05 was considered statistically significant.
For statistical analysis, the numerical values of the variables before and after the intervention in each study group, along with the sample size of each group, were recorded in separate Excel files. Statistical meta-analysis was performed using review manager (RevMan) version 5.4 software (Cochrane Collaboration).
4. Results
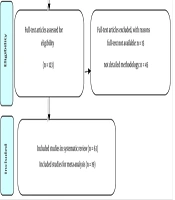
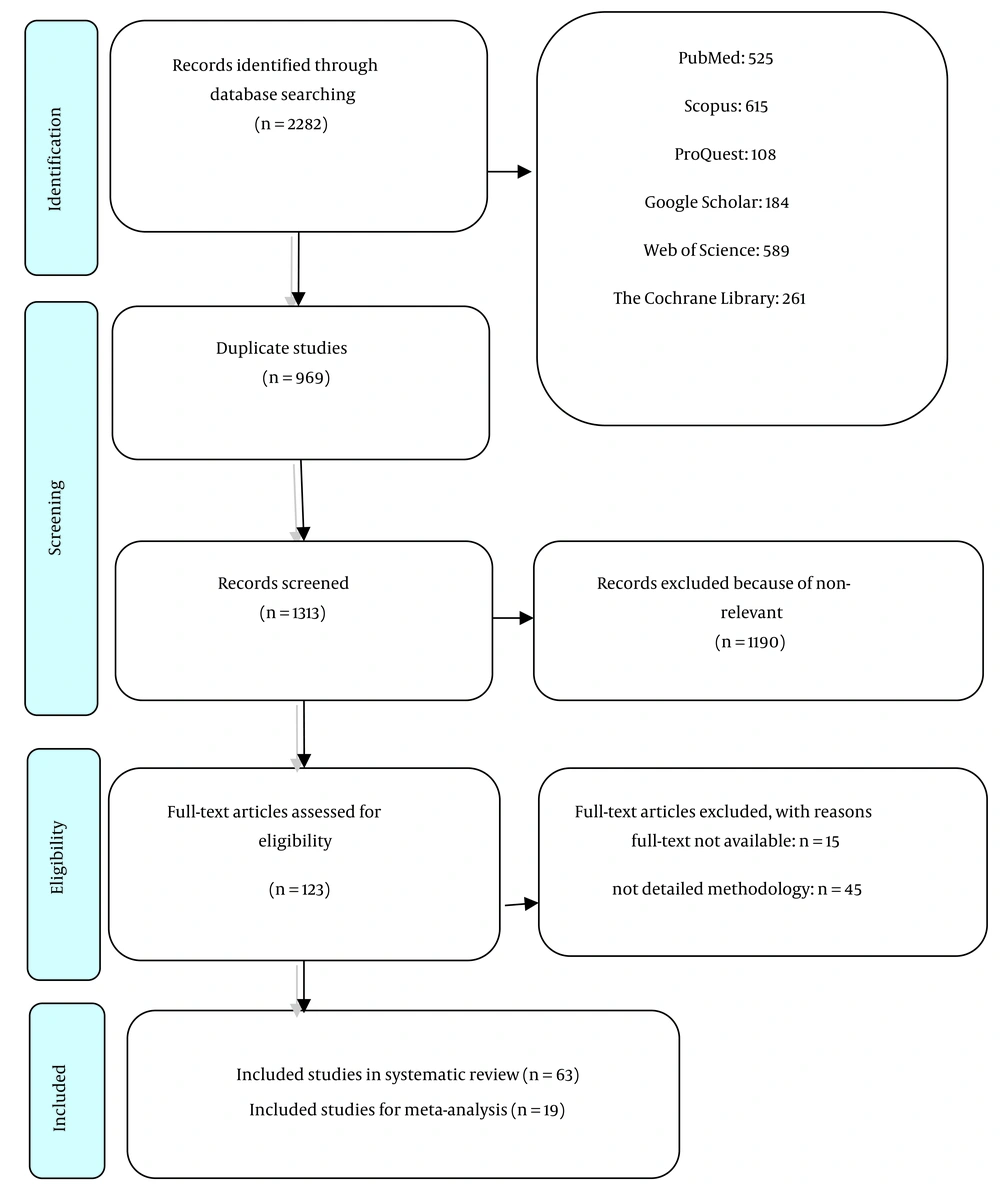
In the initial search of the databases, 2,282 studies were identified. After removing 969 duplicate articles, two independent reviewers screened the titles and abstracts, resulting in a final selection of 63 articles (10-72) . The selection process is depicted in the PRISMA flow diagram (Figure 1). Data extraction tables and a summary of the results are provided in Appendices 2a and b in Supplementary File.
4.1. Voiding Frequency
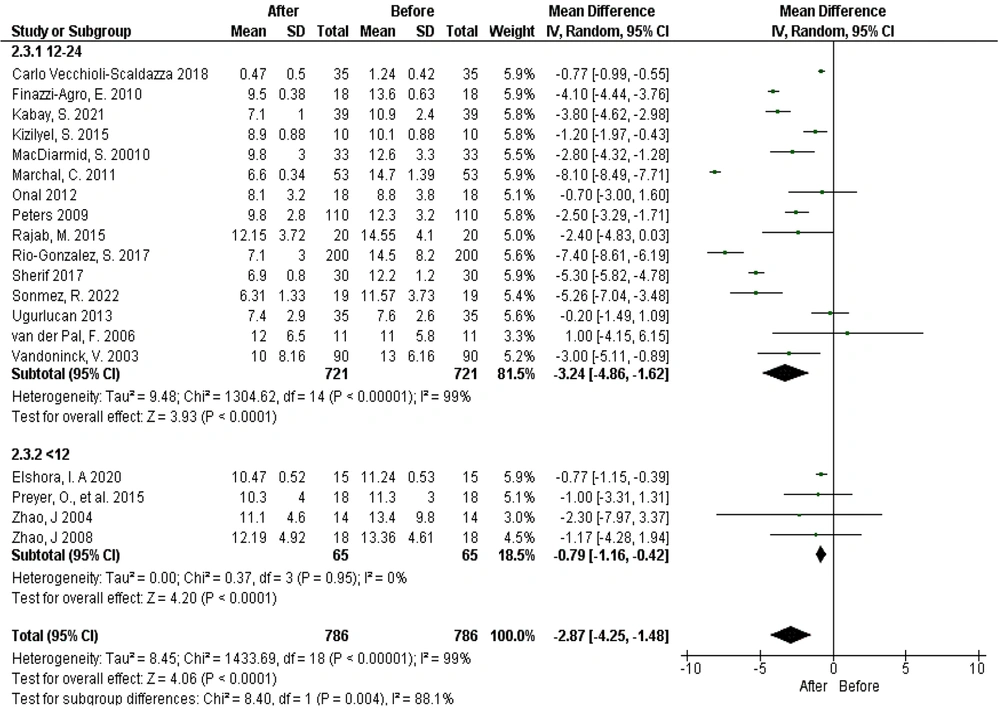
The results showed that in 48 out of 63 studies, the frequency of urination decreased following PTNS. A within-group comparison of average urination frequency using PTNS with the needle method, considering subgroups based on treatment duration (before and after intervention), demonstrated significant heterogeneity among studies (Tau² = 8.45; chi² = 1433.69, df = 18, P < 0.00001, I² = 99%). The mean reduction in urination frequency post-treatment was 2.87 times (95% confidence interval: -4.25 to -1.48, P < 0.0001). This reduction was more pronounced in the 12 - 24 session group (chi² = 8.40, df = 1, P < 0.004, I² = 88.1%). These findings suggest that PTNS with the needle method significantly reduces urination frequency in both the 12 - 24 session and less than 12 session treatment groups (Figure 2).
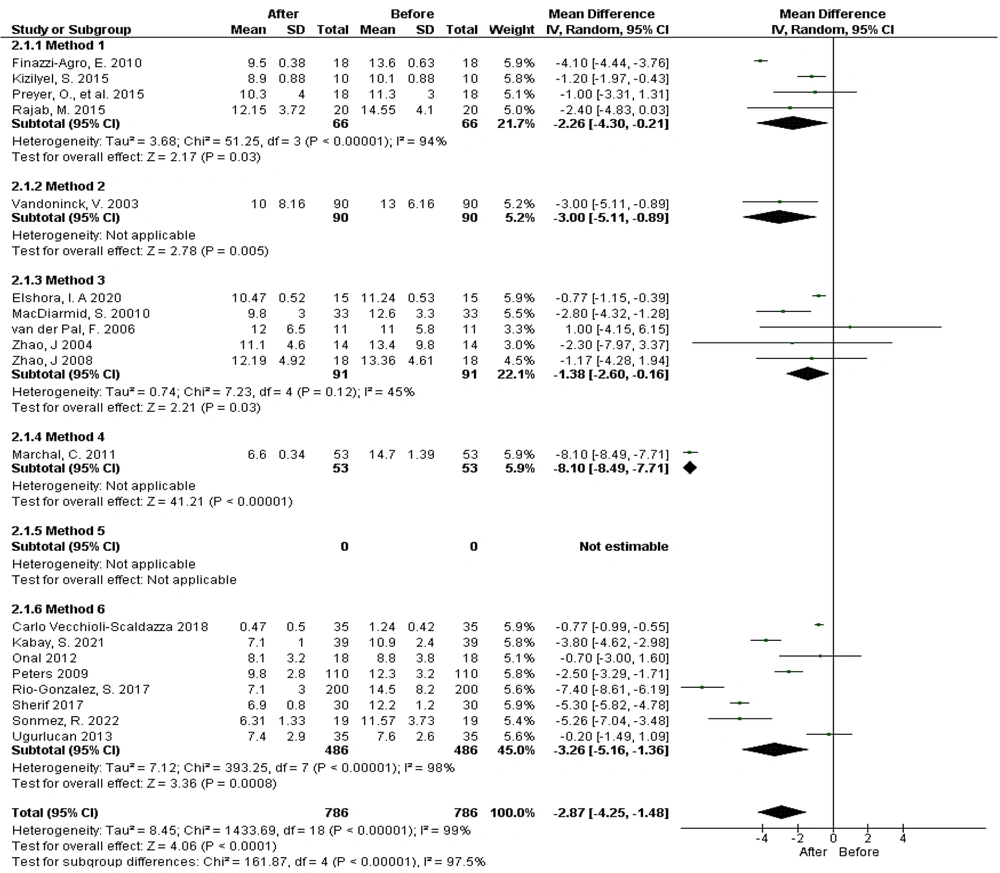
When examining different electrode placement methods, 19 studies were included in the meta-analysis, revealing significant heterogeneity among the studies (Tau² = 8.45; chi² = 1433.69, df = 18, P < 0.00001, I² = 99%). The results indicated that PTNS using the needle method significantly reduces urination frequency with electrode placement methods 1, 2, 3, 4, and 6 (Figure 3).
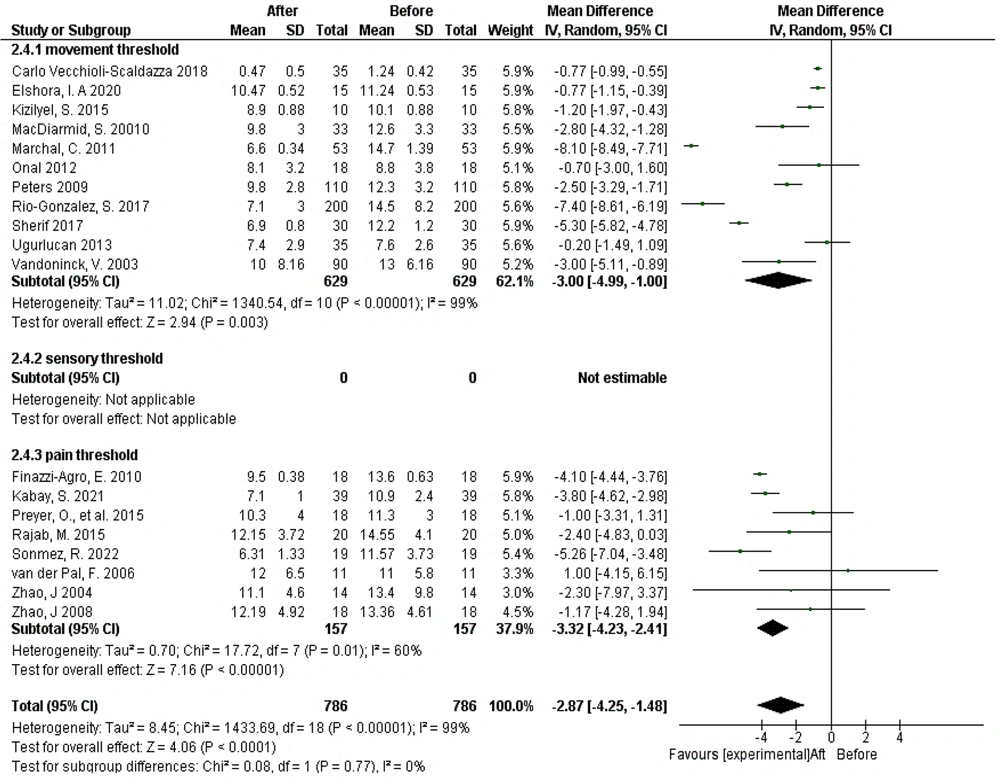
In the subgroup analysis considering stimulation intensity, significant reductions in urination frequency were observed at both the movement and pain thresholds following treatment (Figure 4).
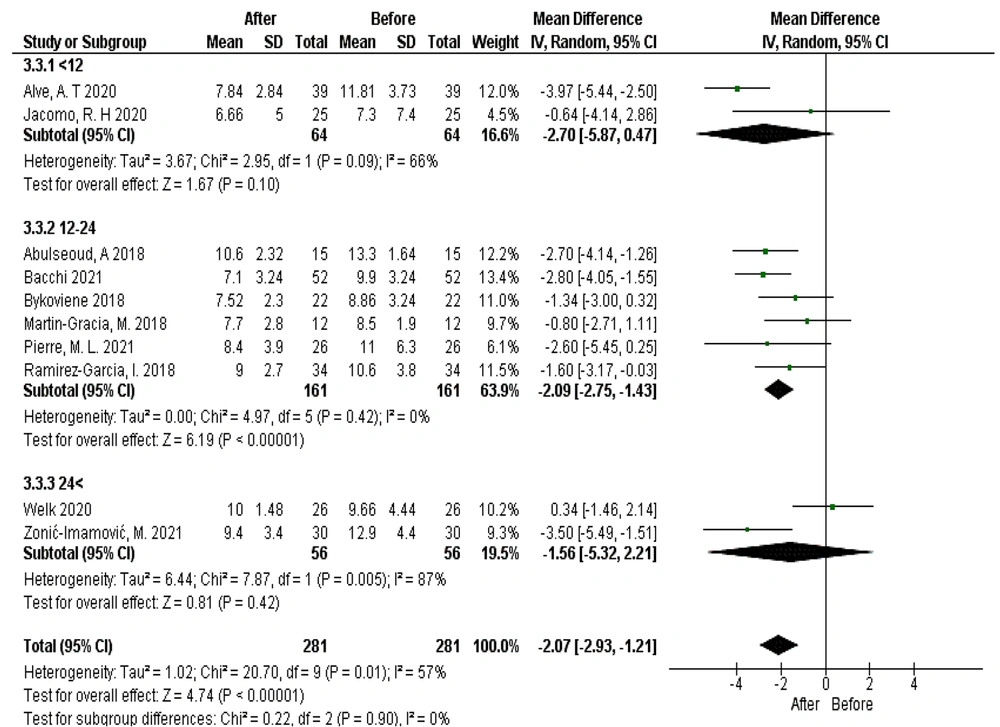
When comparing the average frequency of urination using PTNS with the surface method, accounting for subgroups based on treatment duration (before and after intervention), 10 studies were included in the meta-analysis. After treatment with this stimulation method, the average frequency of urination decreased by 2.07 times (95% CI: -2.93 to -1.21), a statistically significant improvement (P < 0.0001). The decrease in urination frequency was notably greater in the 12 - 24 session treatment period. However, the difference between subgroup values was not statistically significant (chi² = 0.22, df = 2, P = 0.90; I² = 0%) (Figure 5).
When comparing the effect of transcutaneous surface PTNS on mean voiding frequencies, considering electrode placement methods, 8 studies using methods 1, 5, and 6 were analyzed. The heterogeneity among studies was moderate and statistically significant (Tau² = 0.47; chi² = 11.04, df = 7, P = 0.14; I² = 37%). A statistically significant reduction in mean voiding frequencies was observed after treatment with this stimulation method (P < 0.00001). Electrode placement methods 5 and 6 significantly decreased mean voiding frequencies, while method 1 did not produce a significant reduction (Appendix 3 in Supplementary File).
In an analysis of sensory threshold subgroups before and after intervention, 10 studies were included across sensory, movement, and pain threshold subgroups. The heterogeneity among studies was statistically significant (Tau² = 1.02; chi² = 20.70, df = 9, P = 0.01; I² = 57%). There was a statistically significant reduction in mean voiding frequencies following treatment (P < 0.00001). Electrical stimulation intensities at the sensory and pain thresholds led to a significant decrease in mean voiding frequencies, with no statistically significant difference between the subgroups (chi² = 2.25, df = 2, P = 0.33; I² = 11.0%) (Appendix 4 in Supplementary File).
4.2. Mean Voided Volume
In this study, the mean voided volume (MVV) was analyzed as a secondary outcome. Out of the 63 studies reviewed, MVV increased in 15 studies, with only one study reporting a decrease.
For the intragroup comparison of MVV using PTNS with the needle method, 10 studies were included in a meta-analysis, divided into two subgroups: 12 - 24 sessions and more than 24 sessions. There was significant heterogeneity among the studies (Tau² = 309.86; chi² = 47.49, df = 9, P < 0.00001; I² = 81%). The results indicated a statistically significant increase in MVV after treatment with PTNS, with an average increase of 28.28 mL (95% CI: 14.43 to 42.13), and this improvement was statistically significant (P < 0.001, Z = 0.4) (Appendix 5 in Supplementary File).
In the subgroup analysis of electrode placement methods, 10 studies were included in the meta-analysis. MVV significantly increased after treatment compared to pre-treatment values (28.28 mL, 95% CI: 14.43 to 42.13) (P < 0.001, Z = 0.4). The second and third electrode placement methods led to significant increases in MVV, while the first electrode placement method did not show a statistically significant improvement, and no significant reduction was observed with the sixth electrode placement method (Appendix 6 in Supplementary File).
In comparing MVV by the needle method, considering the electrical stimulation threshold limits before and after intervention, 9 studies were included in the meta-analysis. Following treatment, MVV increased significantly by an average of 30.81 mL (95% CI: 17.06 to 44.55) (P < 0.001, Z = 39.4) (Appendix 7 in Supplementary File).
In the intragroup comparison of MVV using the surface method, considering subgroups based on treatment duration before and after intervention, three eligible studies were analyzed across two subgroups: 12 - 24 sessions and more than 24 sessions. The results showed an increase in MVV by 23.58 mL (95% CI: 1.91 to 45.26), which was statistically significant (P = 0.03, Z = 2.13) (Appendix 8 in Supplementary File).
In the intragroup comparison of MVV using the surface method, considering subgroups of electrode placement methods before and after intervention, two studies in the subgroup analysis indicated a mean increase in MVV of 34.32 mL (95% CI: -0.54 to 69.18). This improvement rate was statistically significant (P = 0.05, Z = 1.93) (Figure 1) (Appendix 9 in Supplementary File).
To estimate intragroup changes in MVV before and after intervention using the surface method, based on stimulation threshold, three studies were included across three subgroups: Motor, sensory, and pain thresholds. The improvement in MVV was statistically significant in the motor and sensory threshold subgroups (P = 0.03, Z = 2.13) (Appendix 10 in Supplementary File).
The results for urinary incontinence, urgency, maximum cystometric capacity, and urgency urinary incontinence are summarized in Appendices 11 a - i in Supplementary File.
To assess potential biases, the quality of the selected studies was evaluated using the JBI quality appraisal tool for randomized and quasi-experimental trials, as detailed in Appendix 12 in Supplementary File.
5. Discussion
This systematic review aimed to identify the optimal PTNS application method for treating patients with OAB and painful bladder syndrome by comparing various therapeutic techniques and parameters. Both needle and surface PTNS methods were found to significantly reduce voiding frequency, with the effect being most pronounced with needle stimulation for 12 - 24 sessions and surface stimulation across varying durations. Specific electrode placement methods showed positive outcomes, although effectiveness varied between placements. The greatest benefits were observed at stimulation intensities set to movement and pain thresholds.
In addition to reducing voiding frequency, PTNS—both needle and surface methods—significantly increased MVV, with needle stimulation showing more consistent results. Similar to findings with voiding frequency, certain electrode placements were more effective than others. Stimulation at sensory and pain thresholds also led to a significant increase in MVV. These findings suggest that PTNS, particularly needle-based stimulation, is a promising non-invasive treatment for LUTS, with notable effectiveness in reducing voiding frequency and enhancing MVV.
Micturition is regulated by centers located in the cortex, pons, and spinal cord. The cortical micturition center oversees the regulation of urination, while the spinal micturition center facilitates the completion of the voiding process (1). Elevated anxiety levels can increase central facilitation, leading to a higher frequency and more instances of urinary incontinence. The pontine micturition center plays a crucial role in coordinating and finalizing the voiding process (2).
In adults, the spinal micturition center typically remains inactive, except in cases involving spinal cord injury. Bladder distension triggers low-level vesical afferent activity, which, in turn, stimulates sympathetic outflow to the bladder outlet and pudendal outflow to the external urethral sphincter. This response promotes continence by inhibiting detrusor muscle contraction. Neuromodulation therapy for treating OAB modifies spinal cord reflexes and cortical engagement through afferent signaling, reducing detrusor overactivity and alleviating urinary frequency and urgency. Electrical stimulation applied to the pelvic nerve, sensory fibers of the pudendal nerve, or muscular nerve fibers originating from the lower limbs can effectively inhibit the spinal micturition centers (3).
The tibial nerve, also known as the posterior tibial nerve, originates from the L4-S3 nerve roots and provides innervation to the pelvic floor musculature, bladder sphincters, and the detrusor muscle. Anatomically, it is located approximately three to four centimeters above the medial malleolus. Posterior tibial nerve stimulation is considered a relatively low-risk intervention for OAB, with minor adverse effects such as mild bleeding, discomfort, and skin inflammation at the needle insertion site. However, a standardized treatment protocol for PTNS is not yet established, and various regimens continue to be explored.
Transcutaneous tibial nerve stimulation represents a non-invasive alternative that can be safely performed using surface electrodes placed at the tibial nerve’s anatomical site. Recent studies suggest that both P-PTNS and T-PTNS produce comparable outcomes in managing OAB and bowel dysfunction. A pilot study assessing treatment for fecal incontinence and OAB reported a 53% response rate in both the Global Response Assessment (GRA) and the International Consultations on Incontinence Questionnaires (ICIQ-OAB and ICIQ-LUTS) (3).
While the exact mechanisms by which PTNS achieves its effects remain unclear, several hypotheses have been proposed. Possible mechanisms include changes in brain endorphin levels, depolarization of sacral and lumbar somatic afferent fibers, activation of efferent fibers to the striated urethral sphincter, and cortical reorganization in response to stimulation (66, 69). An analysis of PTNS application methods showed that 33 studies used the P-PTNS method, which was generally more effective (20, 66, 67, 72).
In our systematic review and meta-analysis, no significant side effects, aside from minor transient pain at the needle insertion site, were reported during PTNS treatment (3). In 14 reviewed studies, no adverse effects from PTNS treatment were reported (12, 18, 22, 66, 67). However, 21 studies documented minor side effects, including small blood spots and transient pain, likely associated with the needle insertion (66).
Posterior tibial nerve stimulation devices use biphasic currents, which generally should not produce chemical effects or complications; if chemical effects do occur, they tend to be minimal. No side effects related to the electrical current itself were reported in the studies, likely due to the type and form of the current used.
In the studies reviewed, the stimulation duration per session was consistently set at 20 minutes. However, there was considerable variation in the total number of effective treatment sessions across studies. Both needle and surface PTNS showed greater therapeutic effects over the long term, with low-frequency pulsed electrical stimulation reducing patients’ symptoms through its influence on brain activity (73). Finazzi-Agro et al. found that 12 PTNS sessions could lead to reorganization of cortical networks in supraspinal centers, further supporting PTNS's neuromodulatory effects (74).
Increasing the number of therapy sessions led to more pronounced effects for both transcutaneous and percutaneous stimulation techniques. However, in the 2020 study by Welk, transcutaneous stimulation techniques showed no therapeutic effect when the treatment period exceeded 24 sessions. This suggests that further detailed clinical trials are needed to establish optimal treatment duration for both OAB and painful bladder syndrome.
In 49 studies, posterior tibial nerve stimulation was applied to one leg, while 6 studies applied it bilaterally. When comparing unilateral versus bilateral stimulation in treating painful bladder syndrome, no particular preference was indicated, and no direct comparison was conducted between these two approaches (70, 72).
Patients with OAB experienced a significant reduction in voiding frequency when treated with percutaneous or transcutaneous stimulation on one leg. Further clinical trials are recommended to investigate the effects of bilateral treatment on both legs.
Details regarding specific electrical stimulation parameters, such as current type and form, were generally not reported in the included studies. However, Stoller's method appears to be widely accepted, utilizing parameters including a frequency of 20 Hz, pulse width of 200 µs, and an adjustable pulse intensity of 0 to 10 mA, delivered through a low-voltage device (67, 68, 70). Notably, a pulse duration over 100 µs stimulates sensory fibers and induces analgesia by activating descending pain inhibitory pathways (73).
In comparing electrode placement methods, categorized into six distinct techniques, percutaneous stimulation using methods 2 and 3 significantly reduced voiding frequency and increased MVV in patients with OAB and bladder pain syndrome. This effect may be attributed to the superficial location of the posterior tibial nerve in these regions, allowing for effective stimulation. According to traditional Chinese medicine principles, these acupuncture points are believed to support the spleen and restore balance to yin and blood, as well as to the liver and kidneys. However, minor errors in needle insertion can occur, affecting proper nerve stimulation.
For transcutaneous stimulation techniques, electrode placement method 6 resulted in a significant reduction in voiding frequency and an increase in MVV for overactive bladder syndrome. More clinical trials are recommended to further explore the impact of electrode placement methods in treating OAB and bladder pain syndrome.
5.1. Strengths of the Study
This meta-analysis comprehensively evaluated the effects of different PTNS methods on treating OAB and painful bladder syndrome. The literature search was conducted without restrictions on time or language, ensuring a broad inclusion of relevant studies. Parameters related to the electrical stimulation methods were carefully extracted from the included studies. In cases where complete information was lacking, the study team reached out to corresponding authors to obtain detailed explanations on the PTNS application methods used.
5.2. Weaknesses of the Study
A notable limitation was that 45 out of 63 studies lacked detailed information on PTNS treatment protocols, leading to their exclusion from the meta-analysis. This lack of detail is one of the study's weaknesses. Additionally, some authors did not respond to requests for clarification, limiting the ability to gather comprehensive data.
The studies included in this systematic review exhibited high heterogeneity in terms of PTNS outcomes across both transcutaneous and percutaneous stimulation techniques, as evidenced by a high I² value. For studies examining PTNS for painful bladder syndrome, methodological limitations—including small sample sizes, varied diagnostic criteria, and different stimulation protocols—hindered statistical analysis and meta-analysis.
Further, there was considerable variability in the PTNS application methods used for painful bladder syndrome within intervention groups. The presence of different control groups across studies also complicated efforts to draw conclusive findings on the treatment's efficacy.
5.3. Suggestions
Future clinical trials should aim to reduce the high heterogeneity observed in studies by standardizing parameters where possible. Studies of painful bladder syndrome were not included in the intragroup comparison of the transcutaneous stimulation effect due to methodological limitations, so it is recommended that future trials include this condition. Additionally, evaluating the intensity of stimulation at the sensory threshold, comparing different stimulation thresholds, and rigorously studying electrode placement methods would provide more precise data on optimal PTNS application for these syndromes.
5.4. Conclusions
This study revealed that PTNS can effectively reduce voiding frequency in patients with OAB and painful bladder syndrome. However, due to study heterogeneity and the absence of control groups in many studies, it is challenging to define a standardized PTNS method. Based on the meta-analysis results, PTNS with a frequency of 20 Hz, pulse duration of 200 µs, and stimulation intensity at the pain threshold over 12 - 24 sessions on one foot in both transcutaneous and percutaneous stimulation techniques showed significant reductions in urinary frequency in patients with OAB. While no specific electrode placement method was identified for either transcutaneous or percutaneous techniques, transcutaneous tibial nerve stimulation (TTNS) appears to have minimal side effects.