1. Background
Caesarean section (CS) is a very common surgical procedure worldwide, particularly in developing countries. The CS can reduce the frequency of maternal and newborn mortality (1). Currently, there is global concern about the increasing number of CSs. The CS rate has risen from 4.5% in 1965 to 25% in 1998, exceeding the optimal limit set by the World Health Organization (WHO) (2). In 2004, the CS rate was approximately 30% in the United States. Notably, there has been a substantial increase in the number of CSs in Iran, reported to range between 26% and 87% (3, 4). Several factors contribute to cesarean deliveries: Fetal distress, obesity, dystocia, and advanced maternal age. Moreover, it appears that the primary reasons for this increase are elective cesarean sections and a history of previous CS (5, 6).The CS is one of the most frequently performed surgical procedures; therefore, appropriate selection of the type of anesthesia is of paramount importance. The choice of anesthesia depends on the indication for CS, the safety of the mother and newborn, and the mother's preference (7). Regional anesthesia is generally the preferred technique for CS due to its favorable risk-benefit profile for both the mother and the fetus (8).
In recent years, several medications with various mechanisms of action have been introduced into anesthesiology and intensive care practice. Dexmedetomidine (DX), a highly selective α2-adrenergic agonist acting on the central nervous system (CNS), differs mechanistically from γ-aminobutyric acid (GABAA) receptor agonists (9). The DX is associated with less respiratory depression compared to other sedative agents (10). Additionally, DX mimics natural sleep by acting through endogenous sleep-promoting pathways and can effectively reduce cerebrospinal fluid pressure (11). Alongside its sedative and analgesic effects, DX can lower systemic blood pressure, heart rate, and cardiac output in a dose-dependent manner. Therefore, a dose of 0.25 µg/kg may be appropriate to minimize the risk of hemodynamic instability (12). It has also been demonstrated that only intravenous DX — unlike its spinal or epidural administration — can reduce postoperative nausea and vomiting at doses of 0.5 µg/kg and 1 µg/kg, respectively (13). Notably, DX serves as an alternative to traditional sedatives in critically ill patients and has shown superior efficacy in adults (14). Midazolam, a benzodiazepine, is a GABAA receptor agonist extensively used as a pre-anesthetic sedative in both regional and general anesthesia. It has a rapid onset of action and effectively achieves the desired levels of sedation and anxiolysis. Additionally, midazolam can potentiate the analgesic effects of local anesthetics and has been associated with antinociceptive properties (15). Both DX and midazolam may be considered as adjuvants to spinal analgesia. However, current data comparing these two agents remain inconclusive.
2. Objectives
The aim of this study was to compare the analgesic and sedative effects of DX and midazolam.
3. Methods
3.1. Study Design
This randomized, double-blinded, controlled study was conducted on a total of 135 candidates for CS, classified as American Society of Anesthesiology (ASA) grade I or II. Based on an effect size of 0.4, a statistical power of 0.8, two latent variables, two observed variables, and a confidence level of 95%, the minimum required sample size was calculated to be 44 patients. Accordingly, 45 patients were enrolled in each group. The study was conducted from September 2019 to December 2020 at Kowsar Hospital, affiliated with Semnan University of Medical Sciences.
3.2. Participants
Patients were allocated into intervention groups using block randomization with a parallel sampling method. After applying the inclusion criteria, patients were assigned to one of the three groups according to the randomization list. Inclusion criteria: Women aged 20 to 40 years, undergoing their second to fourth CSs, and candidates for elective CS were enrolled. Exclusion criteria: Participants with a history of hypertension, advanced heart block, hepatic or renal dysfunction, respiratory distress, preeclampsia, Body Mass Index (BMI) > 35, or those taking calcium channel blockers, adrenergic antagonists, or psychotropic drugs were excluded. Additionally, patients undergoing CS lasting longer than 90 minutes were also excluded.
All eligible patients were consecutively enrolled in the study and randomly assigned to one of three groups DX, midazolam (MZ), or normal saline (NS) — according to a pre-determined randomization sequence based on their order of entry. Each group included 45 participants. Randomized permutation blocks of size three and random number tables were used for the random assignment, with Excel software employed to generate the random number tables. The participants, the principal investigator, and the healthcare personnel responsible for outcome evaluation were all blinded to group allocation and the type of intervention administered.
3.3. Ethical Issues
The study was approved by the Ethics Committee (IR.SEMUMS.REC.1397.123) and registered in the Iranian Registry of Clinical Trials (IRCT20151228025732N40). Written informed consent was obtained from all participants prior to their enrollment in the study.
3.4. Intervention
After allocation, participants were prepared for spinal anesthesia in a sitting position. All patients received lactated Ringer’s solution (12 - 15 mL/kg) prior to anesthesia, and oxygen was administered via face mask at a rate of 5 L/min throughout the surgery. Anesthesia was initiated by the slow injection (over 5 seconds) of 2.5 mL of 0.5% bupivacaine using a 14 - 15 gauge needle, in the sitting position. Patients were then promptly placed in the supine position. When the level of anesthesia reached T6, the CS was commenced using a Pfannenstiel incision in the lower part of the uterus. Following delivery and clamping of the umbilical cord, the study medications were administered. In the DX group, patients received 0.5 µg/kg of DX diluted in 100 mL of NS, infused over 10 minutes. The MZ group received 2 mg of MZ in 100 mL of NS over the same time, while the NS group received 100 mL of NS alone. Preparation and administration of the medications were performed by nurses who were blinded to the study design and group allocation. Ephedrine, atropine, and fentanyl were used as needed for the management of hypotension, bradycardia, and breakthrough pain, respectively.
3.5. Measurement of Outcomes
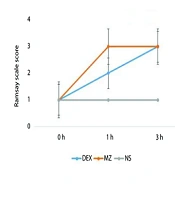
The Ramsay Sedation Scale (RSS) (16) was used to assess the level of sedation at three time points: Prior to anesthesia (0 h), one hour after drug administration (1 h), and three hours after delivery (3 h). The standard RSS scoring is as follows: Score (1) anxious, agitated, or restless; score (2) cooperative, oriented, and tranquil; score (3) responsive to commands only; score (4) brisk response to a stimulus (16).
Adverse effects such as nausea, vomiting, hypotension, shortness of breath, dizziness, and shivering were documented. Pain levels were assessed using the Visual Analog Scale (VAS) at 3, 6, and 12 hours postoperatively. The VAS is a validated tool for measuring both acute and chronic pain. It consists of a 10 cm horizontal line labeled at each end with “no pain” and “worst pain”, on which patients indicate their pain intensity by marking a point on the line (17). In addition, patients were monitored for nausea, vomiting, numbness, and hemodynamic variables over a 24-hour period following surgery.
3.6. Statistical Analysis
The normality of data distribution was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. For quantitative parameters with a normal distribution, parametric tests such as analysis of variance (ANOVA) and independent t-tests were employed. When the distribution was non-normal, non-parametric alternatives including the Mann-Whitney U test and Kruskal-Wallis test were applied. Tukey’s post hoc test was used to analyze differences between subgroups. Qualitative variables were compared using the chi-square test. All statistical analyses were conducted using SPSS version 19, with a significance level set at P < 0.05.
4. Results
4.1. Baseline Characteristics
No patients were lost to follow-up, and all 135 participants were monitored over a 24-hour period. Table 1 presents the clinical characteristics of the participants, with no significant differences observed among the groups. The mean ± standard deviation (SD) of age was 25.6 ± 3.9 years. The patient flow throughout the study is illustrated in Figure 1.
| Characteristics | DEX (N = 45) | MZ (N = 45) | NS (N = 45) | P-Value |
|---|---|---|---|---|
| Age (y) | 25.4 ± 4.3 | 25.1 ± 2.1 | 26.3 ± 3.3 | 0.136 |
| BMI (Kg/m2) | 26.1 ± 2.5 | 27.5 ± 1.5 | 26.9 ± 1.2 | 0.321 |
| Gestational age (wk) | 38.5 ± 0.2 | 38.7 ± 0.1 | 38.5 ± 0.2 | 0.544 |
| Time to reach block (min) | 5.9 ± 1.1 | 5.1 ± 1.2 | 5.5 ± 1.6 | 0.792 |
| Time to recovery from block (min) | 121 ± 21 | 129 ± 25 | 135 ± 19 | 0.365 |
Abbreviations: DEX, dexmedetomidine; MZ, midazolam; NS, normal saline.
a Values are expressed as mean ± SD.
4.2. Levels of Sedation and Analgesia
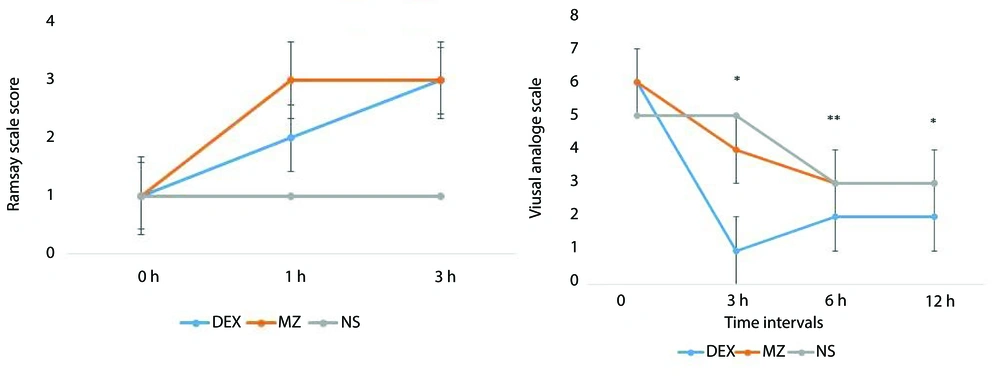
As shown in Figure 2, both the DX and MZ groups achieved the target sedation level (RSS = 2 - 3) compared to baseline values. There were no statistically significant differences between the DX and MZ groups across different time intervals (P = 0.076), although the RSS was slightly higher in the MZ group at the 3-hour mark. Additionally, both treatment groups attained the target analgesia level (VAS ≤ 4). However, between-group differences in pain scores were statistically significant in favor of the DX group at recovery, as well as at the 6-hour and 12-hour intervals (Table 2 and Figure 2).
| Parameters | NS | DX | MZ |
|---|---|---|---|
| Pain | |||
| After 3 hours | 6.53 ± 2.1 | 0.80 ± 1.1 | 6.20 ± 2.2 |
| After 6 hours | 8.20 ± 1.3 | 3.15 ± 1.7 | 8.02 ± 1.1 |
| After 12 hours | 6.31 ± 1.2 | 1.7 ± 1.3 | 6.55 ± 1.4 |
| P-value b | 0.004 | < 0.001 | 0.002 |
| Sedation | |||
| Recovery | 1.24 ± 0.4 | 1.74 ± 0.4 | 1.66 ± 0.5 |
| After 6 hours | 2.08 ± 0.2 | 1.26 ± 0.4 | 2.02 ± 0.2 |
| After 12 hours | 2.00 ± 0.0 | 2.00 ± 0.0 | 1.82 ± 0.4 |
| P-value b | 0.001 | 0.002 | 0.005 |
Abbreviations: DX, dexmedetomidine; MZ, midazolam; NS, normal saline.
a Values are expressed as mean ± SD.
b Kruskal-Wallis, Tukey's post-hoc test.
Repeated measures ANOVA was employed to assess the interaction effect of time on pain intensity and sedation level, both of which were measured at three time points. The analysis revealed that time had a significant impact on changes in both outcomes. Furthermore, the intervention itself was effective, as significant differences were observed among the three groups across repeated measurements. Post-hoc Tukey tests indicated that sedation levels in the DX group were significantly different from those in the NS group (P = 0.037) and the MA group (P = 0.001). However, there was no significant difference in sedation levels between the NS and MA groups (P = 0.478). In terms of pain intensity, the DX group showed significantly lower scores compared to both the NS and MA groups (P < 0.001 for both comparisons). No significant difference in pain intensity was found between the NS and MA groups (P = 0.978) (Tables 3 and 4).
| Parameters | Sum of Squares | df | Mean Square | F | P-Value |
|---|---|---|---|---|---|
| Pain | |||||
| Intercept | 11328.800 | 1 | 11328.800 | 2507.069 | < 0.001 |
| Group | 2320.726 | 2 | 1160.363 | 256.789 | < 0.001 |
| Error | 596.474 | 132 | 4.519 | - | - |
| Sedation | |||||
| Intercept | 1251.714 | 1 | 1251.714 | 9309.489 | < 0.001 |
| Group | 1.872 | 2 | 0.936 | 6.960 | 0.001 |
| Error | 17.748 | 132 | 0.134 | - | - |
| Parameters | Groups | P-Value b | ||||
|---|---|---|---|---|---|---|
| NS | DX | MA | NS vs. DX | NS vs. MA | DX vs. MA | |
| Pain | < 0.001 | 0.978 | < 0.001 | |||
| After 3 hours | 6.53 ± 2.1 | 0.80 ± 1.1 | 6.20 ± 2.2 | |||
| After 6 hours | 8.20 ± 1.3 | 3.15 ± 1.7 | 8.02 ± 1.1 | |||
| After 12 hours | 6.31 ± 1.2 | 1.75 ± 1.3 | 6.55 ± 1.4 | |||
| Sedation | 0.037 | 0.478 | 0.001 | |||
| Recovery | 1.24 ± 0.4 | 1.74 ± 0.4 | 1.66 ± 0.5 | |||
| After 6 hours | 2.08 ± 0.2 | 1.26 ± 0.4 | 2.02 ± 0.2 | |||
| After 12 hours | 2.00 ± 0.0 | 2.00 ± 0.0 | 1.82 ± 0.4 | |||
Abbreviations: DX, dexmedetomidine; NS, normal saline.
a The values are expressed as mean ± SD.
b Post-hoc tests.
4.3. Hemodynamic Variables
The mean ± SD of heart rate (HR), oxygen saturation (SpO2), and blood pressure (BP) for all participants were recorded at recovery and compared with baseline values (Table 5). No significant changes were observed in HR and BP between the DX and NS groups (P > 0.05); however, SpO2 significantly increased in the DX group compared to the NS group (P < 0.05). In the MZ and NS groups, HR and BP did not significantly change following the intervention, although a slight increase was noted (P > 0.05). Notably, BP was significantly higher in the MZ group compared to the NS group (P < 0.05). Furthermore, no significant differences in SpO2 were observed before and after the intervention in the MZ and NS groups (P > 0.05).
| Parameters | Groups | P-Value b | ||||
|---|---|---|---|---|---|---|
| NS | DX | MZ | NS vs. DX | NS vs. MZ | DX vs. MZ | |
| HR | ||||||
| Before intervention | 89.17 ± 8.3 | 96.06 ± 11.4 | 98.95 ± 9.5 | 0.002 | < 0.001 | 0.128 |
| After intervention | 91.35 ± 9.9 | 89.22 ± 6.2 | 95.37 ± 15.9 | 0.227 | 0.156 | 0.014 |
| P-value c | < 0.001 | < 0.001 | 0.002 | - | - | - |
| BP (systolic) | ||||||
| Before intervention | 120.37 ± 8.7 | 111.27 ± 22.2 | 122.35 ± 10.1 | 0.012 | 0.977 | 0.009 |
| After intervention | 103.76 ± 9.0 | 105.81 ± 9.7 | 109.86 ± 11.0 | 0.308 | 0.005 | 0.204 |
| P-value c | 0.006 | 0.018 | 0.008 | - | - | - |
| SpO2 | ||||||
| Before intervention | 98.17 ± 0.8 | 98.53 ± 0.7 | 98.00 ± 2.1 | 0.037 | 0.602 | 0.017 |
| After intervention | 98.26 ± 1.3 | 98.88 ± 0.7 | 97.93 ± 2.5 | 0.010 | 0.441 | 0.009 |
| P-value c | 0.148 | 0.038 | 0.190 | - | - | - |
Abbreviations: NS, normal saline; DX, dexmedetomidine; MZ, midazolam.
a Values are expressed as mean ± SD.
b Post-hoc tests.
c Mann-Whitney U, Tukey's post hoc test.
4.4. Adverse Effects of Treatments
Nausea and vomiting were the most common complications in the DX and MZ groups, reported in 8 cases (17.8%) and 16 cases (35.5%), respectively. Hypotension was more frequently observed in the NS group; however, no significant differences were found in the incidence of hypotension between the MZ and DX groups (Table 6). No participants experienced severe complications leading to death or withdrawal from the study.
| Groups | Complications, Frequency (%) | P-Value a | |||
|---|---|---|---|---|---|
| Nauseas and Vomiting | Hypotension | Dyspnea | None | ||
| NS | 0 | 24 (53.3) | 0 | 21 (46.7) | < 0.001 |
| MZ | 16 (35.5) | 7 (15.6) | 1 (2.2) | 21 (46.7) | |
| DX | 8 (17.8) | 6 (13.3) | 1 (2.2) | 30 (66.7) | |
Abbreviations: NS, normal saline; MZ, midazolam; DX, dexmedetomidine.
a Chi-square test.
5. Discussion
Our study indicated that DX could control pain more effectively than MZ. The intensity of pain in patients who received DX was lower than in those in the MZ group during recovery, as well as at 6 and 12 hours post-operation. The CS is a common surgical procedure during the reproductive years of women (18). Currently, spinal anesthesia is used in the majority of CSs. DX, an α2-adrenergic receptor agonist with well-documented sedative and analgesic effects, primarily exerts its action by inhibiting norepinephrine release at the presynaptic membrane in the subcortical nucleus coeruleus (19, 20). In contrast, MZ acts mainly by inducing sedation at the level of the brain cortex through GABAergic mechanisms (21). Numerous studies have compared different sedative and analgesic agents to identify the most suitable option for CS. In this context, the current study aimed to compare the effects of DX and MZ during CS.
Consistent with our results, Shukla et al. reported that post-operative VAS scores were significantly lower in the DX group compared to the MZ group. In their study, DX was administered intrathecally as an adjuvant agent, which resulted in a longer duration of sensory and motor blockade, as well as prolonged time to first analgesic requirement in patients who received DX. Variables such as sedation level, hemodynamic parameters, and side effects were similar in both groups. Intrathecal DX was introduced as a better adjuvant than MZ due to its ability to extend sensory block duration and reduce the need for postoperative analgesics (22). Additionally, a study by Bi et al. suggested that co-administration of DX with bupivacaine, compared to bupivacaine alone, in patients undergoing cesarean section, prolonged the duration of motor and sensory block and decreased the need for additional doses of lidocaine and fentanyl. Furthermore, DX improved visceral traction responses and abdominal muscle relaxation, while maintaining similar hemodynamic profiles across groups (23). However, Qi et al. reported that intrathecal DX (5 µg) could prolong motor and sensory blockade and provide a comparable analgesic effect to morphine (100 µg) in cesarean section patients (24). It has also been demonstrated that the effect of DX is similar to that of fentanyl in terms of post-operative analgesia (25, 26). Interestingly, DX has shown deeper analgesic effects than morphine (27, 28). Moreover, our results demonstrated that the levels of sedation were not significantly different between the DX and MZ groups. Smiley and Prior showed that the addition of MZ to DX had a similar impact on sedation compared to DX alone; however, patient anxiety and psychomotor performance were reduced. Nonetheless, patients who received MZ experienced amnesic effects and prolonged discharge times (29). The findings of the Smiley and Prior study contrast with our study in some aspects. In our study, DX provided a similar level of sedation compared to MZ, with higher patient satisfaction. In contrast, Smiley et al. reported that patient satisfaction did not increase after MZ administration, which may be attributed to an unpredictable sedative response, making it potentially less suitable for oral surgery procedures (29).
On the other hand, in a study by Kang et al., the sedation score was similar between the two groups who received DX and/or MZ, with no significant differences in systolic blood pressure, pulse rate, O₂ saturation, and body temperature. The results indicated similar hemodynamic effects and patient satisfaction in patients under spinal anesthesia (30). In the current study, post-operative anesthesia complications, including hypotension, nausea, and vomiting, were reported less frequently in patients who received DX. It has been shown that intrathecal administration of DX can reduce the incidence of shivering (20, 31). Moreover, the co-administration of DX and bupivacaine has not been associated with any severe adverse effects or complications (23). Wang et al. reported that the incidence of post-operative nausea and vomiting was lower in the DX group, and the time to recovery of gastrointestinal function was shorter compared to patients who received fentanyl (26). Similarly, Nasseh noted that patients treated with MZ experienced less nausea and vomiting than those who received bupivacaine (32).
5.1. Conclusions
In this trial, we demonstrated that add-on therapy with DX is associated with better pain control in CS compared to MZ. The DX and MZ were similar with respect to their sedative effects.
5.2. Study Limitations
Our findings should be confirmed in larger patient populations and possibly over longer time frames. Moreover, the effects of these drugs on arousability and length of hospital stay could not be assessed.