1. Background
There is undeniable evidence that physical activity can help prevent cardiovascular disease, improve muscle mass and strength, and maintain bone mineral density (1). However, despite its many health benefits, intense physical activity can cause damage to various body tissues due to the increased production of reactive compounds (2). These compounds, known as free radicals, are released as a result of heightened metabolism and increased oxygen consumption (3).
Oxidative stress caused by free radicals has been associated with various diseases, including diabetes mellitus, neurodegenerative disorders (such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis), cardiovascular diseases (including atherosclerosis and hypertension), respiratory diseases (like asthma), cataract formation, rheumatoid arthritis, and various cancers (such as colorectal, prostate, breast, lung, and bladder cancers) (4). Free radicals can react with cellular components, including phospholipid membranes, leading to lipid peroxidation and the formation of products such as malondialdehyde (MDA) (5). Malondialdehyde may then interact with other cellular elements, such as proteins and genomic structures, resulting in extensive cellular damage (6).
Previous research has investigated the impact of various physical activities, including resistance training, on MDA production, yielding mixed results. These disparities can be attributed to factors such as participant characteristics (e.g., age, gender), the specific type of physical activity, and the individual’s exercise history (7, 8).
Additionally, several studies have explored the acute and chronic effects of resistance training on oxidative stress indicators. McBride et al. reported an increase in MDA levels following intense resistance training in resistance-trained men (9). In contrast, Dixon et al. and Deminice et al. found no significant effect of a resistance training session on MDA levels in resistance-trained young men (10, 11). Conversely, studies by Çakir-Atabek et al. (2) and Mardani et al. (12) documented a decrease in MDA levels after 6 to 12 weeks of resistance training. These differing results are likely due to variations in training intensity (13) and the types of subjects studied (14).
The presence of free radicals leads to the destruction of cell membranes, resulting in increased cellular instability. This, in turn, triggers the release of enzymes and intracellular contents, including lactate dehydrogenase (LDH) and creatine kinase (CK), both considered indicators of muscle damage (15, 16). Creatine kinase is recognized as a reliable marker of muscle membrane permeability, as it is found primarily in skeletal muscles and the heart. The destruction of Z-lines and sarcolemma allows the release of this enzyme into the interstitial fluid. Therefore, elevated concentrations of CK in the blood can indicate muscle damage and inflammation (17).
Lactate dehydrogenase is another enzyme found abundantly in the cytoplasm of all body tissues. It plays a crucial role in accelerating the conversion of pyruvate to lactate and vice versa during anaerobic glycolysis (18). Several studies have reported a direct relationship between MDA and CK, both indicators of muscle damage (15, 19, 20). In this context, Spada et al. indicated that intense resistance training damages the skeletal muscle membrane, elevating CK levels for up to 24 hours post-training (21). Akbulut et al. investigated the effect of resistance training on muscle damage indicators in men with no history of resistance training, reporting an increase in CK and LDH levels immediately after exercise (22). Similarly, Gonzalez et al. found elevated LDH levels following a resistance training session at 70% of a one-repetition maximum (1RM) in resistance-trained men (23). However, Motameni et al. observed no changes in LDH and MDA levels but reported an increase in CK following resistance training in women with previous resistance training experience (18). Likewise, Barquilha et al. showed that a resistance training session did not affect CK and LDH serum levels immediately after training (24).
Living organisms are continually exposed to oxidative stress, and they counteract this with both enzymatic and non-enzymatic antioxidant defense mechanisms. Non-enzymatic factors include vitamins A, E, and C, while enzymatic factors involve catalase (CAT), glutathione peroxidase, and superoxide dismutase (25). Each antioxidant plays a distinct role in enhancing the effectiveness of the others, resulting in what is referred to as total antioxidant capacity (TAC). Measuring TAC is critical among antioxidant indicators, as it provides an overall assessment of the body's ability to combat free radicals and maintain antioxidant defenses (26).
Further, CAT is an enzyme that plays a crucial role in reducing oxidative stress. It exhibits two enzymatic activities depending on the concentration of H2O2. Catalase is a key enzyme that protects cells against oxidative damage caused by hydrogen peroxide (27). Earlier studies have highlighted the effectiveness of endurance activities in increasing CAT levels. However, fewer studies have explored the effects of resistance training on antioxidant enzymes compared to endurance training (28). In this context, Park and Kwak reported an increase in TAC after seven weeks of endurance and resistance training in young men (29). Similarly, Azizbeigi et al. observed an increase in superoxide dismutase (SOD), a decrease in MDA, and no change in plasma TAC following resistance training in young men with no prior resistance training experience (26). However, some studies have shown positive effects of resistance training on antioxidant enzyme levels, including CAT, in adults (30).
Resistance exercises can be performed in various styles, including traditional, circuit, and superset formats, with each exercise form and rest time potentially yielding different physiological effects. Supersets are resistance training exercises that pair two movements targeting either the same muscle group (agonist/compound superset) or opposing muscle groups (antagonist/reciprocal supersets) (31). Agonist supersets can be executed in two forms: Post-exhaustion and pre-exhaustion. In post-exhaustion supersets, a basic, multi-joint movement (recruiting multiple muscle groups) is performed first with maximum resistance, followed by a single-joint movement (recruiting a single muscle group). In pre-exhaustion supersets, a single-joint movement is performed first, exerting pressure on a specific muscle group at near-maximum resistance. Then, a multi-joint movement is executed, targeting the same and other muscle groups until exhaustion (32). Additionally, Soleymani et al. demonstrated that a superset resistance training session involving both agonist and antagonist muscle groups significantly increased CK levels in trained young men (33).
According to previous research, it can be concluded that intensity, duration, type of physical activity, and fitness levels have varying effects on oxidative stress, muscle damage, and the antioxidant system (8). While athletes frequently use two distinct methods for performing superset exercises targeting agonist muscle groups, existing research has yet to examine the differential motor unit recruitment caused by these protocols or their potentially varying effects on oxidative stress, muscle damage, and antioxidant responses.
2. Objectives
Therefore, there is a need for a study that investigates the impact of these two training methods on (1) oxidative stress markers; (2) muscle damage; and (3) antioxidant capacity, while also comparing the effects of these two protocols in resistance-trained men.
3. Methods
A quasi-experimental study with a pretest-posttest design was conducted. The study was scientifically and ethically approved by the Specialized Sports Physiology Committee of the University of Bojnord (Iran) and by the Ethics Committee in Biological Sciences Research of the University of Bojnord (IR.UB.REC.1401.011).
3.1. Participants
The statistical population consisted of healthy young male athletes from Bojnord, aged 20 to 30 years, who had at least two years of regular resistance training experience. The sample size was calculated to be 12 participants using G*Power 3.0.10 (University of Düsseldorf, Germany), assuming a significance level of 5% (α = 0.05) and a power of 80% (β = 0.2). Subjects were selected through invitations and online notifications. The inclusion criteria comprised men who had been engaging in resistance training (at least three sessions per week, each lasting 60 minutes for two years), the ability to perform at least one repetition of a squat with a resistance of 1.25 times their body mass, and the ability to perform one repetition of a chest press with a resistance equal to their body mass (33). Exclusion criteria included any history of anabolic steroid supplement use, antioxidant supplement intake, or failure to actively participate in the study protocol.
Once the subjects were informed of the study objectives, procedures, potential benefits, and risks, they provided written informed consent before participation. Two individuals were removed from the study for failing to adhere to the protocol or participate in blood sampling. The characteristics of the subjects are shown in Table 1. All participants were given the right to withdraw from the study at any stage and were assured that their medical and sports information would be kept confidential and used only for research purposes.
| Variables | Mean ± SD |
|---|---|
| Age (y) | 24.25 ± 4.92 |
| Height (cm) | 175.12 ± 6.19 |
| Weight (kg) | 74.75 ± 8.44 |
| BMI (kg/m2) | 24.04 ± 1.64 |
3.2. Determination of One Repetition Maximum
Prior to the training program, the maximum number of pull-ups and parallel bar dips were tested, along with 1RM tests for squats, deadlifts, seated leg extensions, lying leg curls, biceps curls, and triceps curls. A standardized 15- to 20-minute warm-up, consisting of submaximal aerobic and stretching exercises, was performed before each test. The 1RM was typically determined within 3-6 trials using Epley’s equation (34). All testing procedures were closely supervised, maintaining an instructor-to-subject ratio of 1:1, and verbal encouragement was provided to all participants. All measurements were conducted with consistent body positioning, using the same resistance equipment, and administered by the same test administrator. Subjects were asked to perform glycogen loading for three days prior to the 1RM tests (Table 2).
| Exercise | 1RM |
|---|---|
| Squat | 117 ± 14.75 |
| Deadlift | 121.5 ± 16.16 |
| Parallel bar dip | 15.80 ± 2.74 |
| Pull-up | 13.50 ± 184 |
| Seated leg extension | 70.50 ± 6.32 |
| Lying leg curl | 50 ± 7.35 |
| Triceps curl | 32.10 ± 2.37 |
| Biceps curl | 43 ± 8.23 |
Abbreviation: 1RM, 1-repetition maximum.
3.3. Resistance Training Protocol
Three days after determining their 1RM, the subjects performed two types of supersets, with a 7-day interval between sessions, at the University gymnasium. For the postexhaustion supersets, participants completed 10 repetitions of multi-joint movements at 80% of their 1RM, followed immediately by single-joint movements at 80% of their 1RM until exhaustion. For the preexhaustion supersets, participants first performed 10 repetitions of single-joint movements at 80% 1RM, followed immediately by multi-joint movements at 80% 1RM until exhaustion. The multi-joint movements included squats, deadlifts, parallel bar dips, and pull-ups, while the single-joint movements included seated leg extensions, lying leg curls, biceps curls, and triceps curls. Each muscle group followed a training program of three sets with a 90-second rest between sets and a 3-minute rest between different movements. Participants were prohibited from engaging in intense physical activity, taking medication, or consuming alcohol. All research activities took place at 25°C and 50 - 55% humidity. Athletes had unrestricted access to water during the exercise protocol. To balance training effects, half of the participants performed postexhaustion supersets in the first session, while the other half completed preexhaustion supersets. In the second session, held one week later, the training method was switched. The food plan for all participants was identical and followed the university’s nutrition guidelines.
3.4. Blood Collection and Biochemical Analyses
After a 10-hour fasting period, a 5-mL blood sample was collected from the antecubital vein before and 24 hours after each training session. The blood samples were centrifuged for 5 minutes at 3000 rpm and 4°C to separate the serum (35). Lactate dehydrogenase and CK levels were measured using a commercial kit (Pars Azmoun, Karaj, Iran) through a spectrophotometric method, with sensitivities of 5 and 4 international units per liter, respectively. Malondialdehyde levels were determined using the Lapenna method with absorbance spectrophotometry at 535 nm (36). Total antioxidant capacity was measured using the Benzie and Strain method (37), and CAT levels were assessed using the method presented by Hadwan and Abed (38).
3.5. Statistical Analysis
The data were analyzed using the SPSS statistical package for Windows, version 16.0 (SPSS, Chicago, IL). Data are presented as mean ± standard deviation. The Shapiro–Wilk test was first used to assess the normality of data distribution. A paired t-test was conducted to determine mean differences between pre- and post-intervention values for all variables. An independent sample t-test was used to compare all variables between the two groups at baseline (pretest). To assess the effect of superset training on various parameters (MDA, CK, LDH, TAC, CAT), an independent t-test was performed to compare pre- and post-training values for each variable between the two groups. P-values less than 0.05 were considered statistically significant for all tests.
4. Results
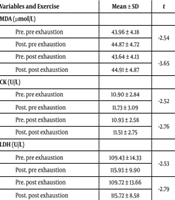
Based on the paired t-test results (Table 3), all oxidative stress, muscle damage, and antioxidant indices (MDA, CK, LDH, TAC, CAT) increased significantly compared to baseline levels (P-value ≤ 0.05) in response to both superset protocols. Additionally, the results revealed no significant difference between the effects of the two types of superset resistance training on MDA, CK, and LDH levels (P-value > 0.05). However, there was a significant difference between the acute effects of the two protocols on TAC and CAT indices (P-value ≤ 0.05). Specifically, the postexhaustion superset caused a greater increase in TAC and CAT compared to the preexhaustion superset in healthy young resistance-trained men.
| Variables and Exercise | Mean ± SD | t | Sig. | Mean Difference Between Protocols | SD | t | Sig. |
|---|---|---|---|---|---|---|---|
| MDA (µmol/L) | 0.0358 | 0.862 | 1.313 | 0.222 | |||
| Pre. pre exhaustion | 43.96 ± 4.18 | -2.54 | 0.031 a | ||||
| Post. pre exhaustion | 44.87 ± 4.72 | ||||||
| Pre. post exhaustion | 43.64 ± 4.13 | -3.65 | 0.005 a | ||||
| Post. post exhaustion | 44.91 ± 4.87 | ||||||
| CK (U/L) | 0.237 | 1.272 | 0.058 | 0.570 | |||
| Pre. pre exhaustion | 10.90 ± 2.84 | -2.52 | 0.030 a | ||||
| Post. pre exhaustion | 11.73 ± 3.09 | ||||||
| Pre. post exhaustion | 10.93 ± 2.58 | -2.76 | 0.022 a | ||||
| Post. post exhaustion | 11.51 ± 2.75 | ||||||
| LDH (U/L) | 0.0410 | 2.850 | 0.454 | 0.661 | |||
| Pre. pre exhaustion | 109.43 ± 14.33 | -2.53 | 0.032 a | ||||
| Post. pre exhaustion | 115.93 ± 9.90 | ||||||
| Pre. post exhaustion | 109.72 ± 13.66 | -2.79 | 0.021 a | ||||
| Post. post exhaustion | 115.72 ± 8.58 | ||||||
| TAC (Mmol/L) | 0.013 | 0.014 | 2.750 | 0.022 a | |||
| Pre. pre exhaustion | 1.21 ± 0.045 | -3.67 | 0.005 a | ||||
| Post. pre exhaustion | 1.22 ± 0.054 | ||||||
| Pre. post exhaustion | 1.20 ± 0.043 | -4.43 | 0.002 a | ||||
| Post. post exhaustion | 1.23 ± 0.048 | ||||||
| CAT (µmol/L) | 1.300 | 1.636 | 2.512 | 0.033 a | |||
| Pre. pre exhaustion | 24.40 ± 3.62 | -3.67 | 0.005 a | ||||
| Post. pre exhaustion | 25.3.6360 ± | ||||||
| Pre. post exhaustion | 23.80 ± 3.04 | -7.31 | 0.000 a | ||||
| Post. post exhaustion | 26.30 ± 3.19 |
Abbreviations: MDA, malondialdehyde; CK, creatine kinase; LDH, lactate dehydrogenase; TAC, total antioxidant capacity; CAT, catalase.
a A significant difference at the level of P ≤ 0.05.
5. Discussion
The research findings revealed that performing superset resistance training of agonist muscle groups, using both pre-exhaustion and postexhaustion methods, led to an increase in oxidative stress markers (MDA), muscle damage markers (LDH, CK), and antioxidant markers (CAT, TAC) in resistance-trained men. Additionally, the results indicated no significant difference between the effects of these two training methods on oxidative stress and muscle damage markers. However, postexhaustion superset training enhanced antioxidant markers (CAT, TAC) more significantly in resistance-trained men.
Despite the beneficial effects of regular exercise on overall wellness, evidence suggests that intense physical activity, including resistance training, can increase the production of free radicals and oxidative stress in the body (20). Adaptations in individuals with a history of resistance training can influence their physiological responses to training. Another factor influencing these responses is the type of resistance training performed (39). Therefore, this study aimed to compare the acute effects of two different agonist muscle superset resistance training protocols on oxidative stress, muscle damage, and antioxidant markers in young, resistance-trained men.
The results showed that both superset protocols (postexhaustion and preexhaustion) increased MDA levels in young resistance-trained men, with no significant difference between the two groups. Given the conflicting findings of previous studies, the current investigation aligns with McBride et al.’s study (9), which also showed an increase in oxidative stress following a resistance training session. However, the results differ from studies reporting no increase or a decrease in MDA following a resistance training session (2, 10-12).
Individual differences and training intensity are important factors affecting physiological responses to training, and these may explain the contradictory results compared to the aforementioned studies. Moreover, traditional methods of resistance training (8 to 10 repetitions at 75 to 80 percent of 1RM) have been used in studies with inconsistent findings (2, 10, 11). Thus, the type and intensity of the resistance training protocol may be the primary reasons for these discrepancies. Additionally, the subjects in this study were individuals with high levels of physical fitness and a history of resistance training, which could further explain the variations in results due to differences in fitness levels among participants.
Two theories propose explanations for the increased production of free radicals in muscles during resistance exercise. The first, widely supported, is the ischemia-reperfusion mechanism theory (9). This theory suggests that resistance exercise, marked by intense muscle contractions and subsequent vascular occlusion (pressure on blood vessels), leads to a temporary reduction in blood flow and oxygen delivery to the muscles. During the rest period, previously ischemic tissues are reperfused, causing a surge in oxygen availability. This reoxygenation is thought to trigger the production of free radicals, which in turn initiate muscle adaptation through a process known as mechanistically activated differentiation. The second theory attributes the increase in free radicals to heightened mechanical stress. According to this theory, high forces generated during the eccentric phase of muscle contraction result in tissue damage and inflammation, both of which contribute to free radical production and lipid peroxidation (40).
Our findings suggest that, regardless of the type of superset resistance training, both postexhaustion and preexhaustion supersets increase oxidative stress in young resistance-trained men, with this effect persisting for at least 24 hours post-training. Furthermore, the study demonstrated that both types of agonist muscle superset resistance training elevate muscle damage markers (CK and LDH) in this population. These results are consistent with prior research (21-23). However, our findings differ from those of Barquilha et al. and Motameni et al. (18, 24), who reported no significant effect of resistance training on muscle damage markers. The discrepancy may be due to differences in exercise type and participant gender, as Barquilha’s (24) study employed an isometric resistance training protocol, and Motameni’s study focused on female participants.
Additionally, this study revealed an increase in MDA, CK, and LDH levels following a superset resistance training session, which supports previous findings linking oxidative damage to muscle damage markers (15, 19, 20). It has been suggested that the surge in free radicals following high-intensity resistance training disrupts cell stability by damaging the cell membrane, leading to the release of enzymes such as CK and LDH (18, 22, 23).
The results of the present study also revealed that superset resistance training, in both protocols, increased TAC and CAT levels in young resistance-trained men. This finding aligns with the results reported by Park et al. but contradicts the findings of Azizbeigi et al. (26, 29). The discrepancies between outcomes may stem from variations in exercise intensity. Azizbeigi et al. (26) utilized a moderate-intensity protocol, whereas the present study focused on high-intensity exercise. Moreover, a significant difference was observed between the two types of superset resistance training in terms of their effects on antioxidant indices. The postexhaustion superset training resulted in a greater increase compared to the preexhaustion method. Based on these findings, it can be suggested that a superset protocol starting with multi-joint exercises followed by single-joint exercises to exhaustion may be more effective at enhancing antioxidant capacity than a protocol sequencing single-joint exercises before multi-joint ones.
A pertinent question arises from this study: Given that the findings demonstrate improved antioxidant capacity in human skeletal muscle after both forms of agonist superset resistance training, can the observed increase in muscular antioxidant indices serve a protective role against oxidative damage induced by reactive oxygen species within muscle fibers? As indicated by the results, despite the rise in CAT and TAC after resistance training, markers of muscle damage and oxidative stress remained elevated compared to pre-training values even 24 hours after the workout. These findings suggest that while exercise-induced improvements in antioxidant capacity occur, they are not sufficient to completely offset oxidative stress or prevent the elevation of muscle damage markers within the first 24 hours post-exercise. This effect seems to be independent of the specific superset resistance training protocol used. However, the data show that postexhaustion superset training has a greater effect on enhancing enzyme activity and antioxidant capacity.
While the study controlled for dietary intake by providing a standardized diet throughout the research period, genetic predispositions, lifestyle factors, and the psychological states of the subjects were not controlled. As the study participants were young, resistance-trained men, it is recommended that future research examine the effects of such training on oxidative stress, muscle damage, and antioxidant markers in resistance-trained women. Additionally, the long-term effects of agonist muscle supersets in individuals with a history of resistance training should be explored.