1. Context
Endometriosis is a chronic condition in women characterized by the abnormal growth of endometrial tissue outside the uterine cavity or myometrium (1). This condition is associated with infertility, chronic pelvic pain, and asymptomatic presentations in 31%, 42%, and 23% of cases, respectively, among women of reproductive age (2). Deep infiltrating endometriosis (DIE) is a severe form of endometriosis, defined by the infiltration of endometrial-like tissue into the deeper layers of the pelvic organs and tissues (3). Deep infiltrating endometriosis typically involves specific areas such as the rectovaginal septum, uterosacral ligaments, pararectal space, and vesicoureteral fold. However, it may also affect the rectum, sigmoid colon, ileum, ureter, diaphragm, and other less common locations (4). Among symptoms, dysmenorrhea is the most frequent type of pain experienced by women with endometriosis (5, 6). Deep infiltrating endometriosis is also strongly associated with pelvic pain and dyspareunia (7).
Medical managementof endometriosis includes treatments such as danazol, progesterone medications, gestrinone, combined estrogen and progesterone formulations, gonadotropin-releasing hormone agonists, and other comparable options (8). However, surgical intervention remains the most effective approach for managing DIE (9) due to the limitations of medical therapy in controlling symptoms. Studies have shown that while surgery can significantly alleviate pain, there remains a risk of disease recurrence across all stages of the condition. Various laparoscopic approaches have been utilized for the treatment of bowel endometriosis, including shaving, disc excision, and segmental resection (10). However, no definitive evidence has established the superiority of one surgical technique over another, as limited medium-term studies compare safety, effectiveness, and recurrence rates among these techniques (11).
Recurrence is defined as the reappearance of symptoms and signs following treatment and remission and varies depending on the duration of follow-up (12). Evidence suggests that surgery alone can effectively control pain caused by endometriosis across all stages of the disease. On the other hand, the effectiveness of treatment in women with endometriosis is often measured by reductions in pain and improvements in infertility following treatment (13).
Given the increasing prevalence of endometriosis in recent decades, addressing the knowledge gap in current review studies and updating existing information is essential.
2. Objectives
This systematic review and meta-analysis aim to investigate the recurrence and pregnancy rates following surgical treatment of DIE in reproductive-age women. Additionally, the study evaluates the preoperative and postoperative prevalence of common accompanying symptoms in these cases.
3. Data Sources
3.1. Study Design and Registration
This investigation was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) framework. The PRISMA guidelines include a total of 27 components, covering various aspects of systematic reviews and meta-analyses, such as abstracts, methods, results, discussions, and the disclosure of financial resources (14).
This study was approved by the ethical code IR.ABZUMS.REC.1401.025 at Alborz University of Medical Sciences. Furthermore, it was registered on the PROSPERO website under the ID "CRD42022328051."
3.2. Search Strategy
PubMed, Web of Science, Scopus, Google Scholar, Cochrane Library, and ProQuest were systematically searched from 2010 to August 25, 2024. Initially, each keyword was searched individually, followed by their combination using "AND" or "OR" to create new keywords or phrases. The search strategy, employing MeSH keywords, is outlined below:
'Deep endometriosis'[tiab] OR, 'Deep infiltrating endometriosis'[tiab] OR, 'DIE'[tiab], OR 'Bowel endometriosis'[tiab] OR, 'Colorectal endometriosis'[tiab] OR, 'Rectovaginal endometriosis'[tiab] OR, 'Bladder endometriosis'[tiab] OR, 'Ureteral endometriosis'[tiab] OR, 'Diaphragmatic endometriosis'[tiab], OR 'Endometrioma'[tiab], OR 'Endometriomas'[tiab], AND 'Surgery'[tiab], OR 'Surgery treatment'[tiab], AND 'Recurrence'[tiab], OR 'Recrudescence'[tiab], OR 'Recrudescences' [tiab], OR, 'Relapse'[tiab], 'Relapses'[tiab], AND 'Fertility rate' [tiab], OR 'pregnancy rate'[tiab].
3.3. Eligibility Criteria
Eligibility criteria were established based on the PICO-S framework, where P represents the population (reproductive-age women), I represents the intervention (surgical procedures), C represents the comparison (without comparison), O represents the outcome (recurrence and pregnancy rates), and S represents the study design [cohort, cross-sectional, and randomized clinical trials (RCTs)]. Studies published up to August 25th, 2024, with full-text availability in English or Persian, were included. Exclusion criteria comprised letters, comments, short communications, conference abstracts, grey literature, review studies, and other irrelevant studies.
3.4. Study Selection
To achieve the final results presented in Table 1, a systematic process was initiated. The titles and abstracts of all retrieved studies were screened based on the inclusion criteria. In the next step, the full texts of the eligible abstracts were evaluated, and if the full text was inaccessible, an email was sent to the corresponding author. Subsequently, the full texts of eligible studies were thoroughly examined according to the specified criteria, and relevant studies were selected for analysis. This process was conducted independently by two reviewers, and any disagreements were resolved through discussion. In cases where the study content was unclear, the authors were contacted directly for clarification.
| ID | Author | Year | Country | Design | Number of Participants | Age (y) | BMI (kg/m2) | Symptoms | Location of Endometriosis | Surgical Techniques | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Missori, et al. (15) | 2024 | Spain | Cohort | 103 | 36.55 (23 - 50) | 24.66(15.90 - 33.59) | Dyspareunia; dysmenorrhea; chronic pelvic pain; dyschezia; stranguria; abdominal distension; tenesmus; constipation; diarrhea; hematochezia | Intestine | Bowel resection (sigmoid-rectum resection, rectal shaving, discoid resection, ileal resection, strictureplasty) | 27.52 (1 - 54) |
| 2 | Han and Zheng (16) | 2024 | China | Cohort | 212 | 28.90 ± 6.010 | 23.03 ± 3.625 | Severe dysmenorrhea | Ovaries | Laparoscopic surgery | 24 |
| 3 | Zhang, et al. (17) | 2023 | China | Cohort | 63 | 31.25 ± 5.81 | 22.62 ± 2.79 | Pain; urinary symptoms; gastrointestinal symptoms; infertility; adenomyosis | Pelvis | Transumbilical single-port laparoscopy | 22.90 ± 5.46 |
| 4 | Yang, et al. (18) | 2023 | China | Cross-sectional | 347 | 35.18 ± 6.187 | Not mentioned | Dysmenorrhea; Adenomyosis | Ovaries | Not mentioned | 1 - 60 |
| 5 | Leborne, et al. (19) | 2022 | France | Cohort | 165 | 34.00 (IQR: 11.00) | 23.00 (IQR: 6.00) | Dysmenorrhea; dyspareunia; pain when defecating | Uterus, ovaries, fallopian tube, pelvis peritoneum, vagina, recto vaginal wall, bowel and cutaneous scar | Surgical excision | 1.5 |
| 6 | Zhang et al. (20) | 2022 | China | Cohort | 34 | 30.22 ± 3.62 | Not mentioned | Primary or secondary infertility | Ovaries | Minimally invasive surgical techniques | 26.57 ± 14.51 |
| 7 | Kim et al. (21) | 2022 | South korea | Cohort | 56 | 36.4 ± 5.7 | 21.9 ± 4.6 | Palpable abdominal mass with increasing in size during previous year | 55.6% C/S scar; -5.6% episiotomy site; -16.7% inguinal area; -22.2% laparoscopic trocar site (including umbilicus) | Local excision ; in metastatic cases laparoscopic hysterectomy with bilateral salpingo-oophorectomy with pelvic lymph node dissection | 31.8 ± 26.9 |
| 8 | Roman et al. (22) | 2022 | France | Cohort | 55 | 27 - 36 | Not mentioned | Dysmenorrhea; deep dyspareunia; pelvic pain outside periods | Rectum | Segmental resection; nodule excision via shaving or disk excision | 84 |
| 9 | Ceccaroni, et al. (23) | 2022 | Italy | Cohort | 703 | Median: 36 years (range: 21 - 56) | 22.7 ± 4.9 | Chronic pelvic pain; dysmenorrhea; dysuria; dyspareunia; dyschezia | Bowel | Laparoscopic bowel shaving with concomitant radical excision of DIE | Median: 14 months (range: 6 - 49) |
| 10 | Sarbazi, et al. (24) | 2021 | Iran | Cohort | 174 | 34.86 ± 6.47 | 24.95 ± 4.40 | MenorrhagiaMetrorrhagia; dysmenorrhea; dyspareunia; irregular menstruation; infertility | Ovarian fossa and vaginal vault | Laparo¬scopic surgery | 48 |
| 11 | Yela, et al. (25) | 2021 | Brazil | Cohort | 72 | 39.7 ± 6.3 | 26.9 ± 5.0 | Dysmenorrhea; dyspareunia; chronic pelvic pain; dyschezia; dysuria; infertility | Intestinal tract, urinary tract, ovaries, uterine/bladder pouch, douglas pouch | Surgical treatment to remove endometriosis lesions | 4.56 ± 2.60 years |
| 12 | Vidal, et al. (26) | 2021 | France | Cohort | 50 (early group = 25 & late group =25) | Early group: 31.7 ± 3.9 & late group : 34.0 ± 3.5 | Early group: 24.0 ± 4.3 & late group : 22.6 ± 3.5 | Infertility; pelvic pain; dysmenorrhea; dyspareunia; pain on defecation; urinary symptoms | Bowel | Laparoscopic removal of deep endometriosis lesions | 34.1 |
| 13 | Parra, et al. (27) | 2021 | Brazil | Cross-sectional | 77 | 36.4 ± 5.5 | 25.7 kg/m2 (min-max:17.9 - 37.5) | Infertility; dyspareunia; dysmenorrhea adenomyosis | Bowel | Laparoscopic discoid resection, segmental resection, or shaving of DIE | 2.3 years (6 mo-6.5 years) |
| 14 | Jayot, et al. (28) | 2021 | France | Cross-sectional | 93 | 34 (range:19 - 59) | 23 (range:17 - 37) | Dysmenorrhea; dyspareunia chronic pelvic pain; dyschezia painful defecation infertility | Colorectal | Discoid colorectal resection | 20 |
| 15 | Abesadze, et al. (29) | 2020 | Germany | Cohort | 15 | RVE: 34 ± 5.4; RCE: 31 ± 4.8 | Not mentioned | Cyclic pelvic pain; chronic pelvic pain; dyspareunia; dyschezia; dysuria; infertility | RVE & RCE | Single laparoscopy was performed in RCE patients & vaginal assisted laparoscopy in RVE patients | 36 |
| 16 | Ceccaroni, et al. (30) | 2020 | Italy | Cohort | 264 | 36.8 ± 5.6 | 21.03 ± 3.26 | Urinary frequency; tenesmus; hematuria; dysmenorrhea; pelvic pain; dyspareunia; dysuria; dyscheziacyclic sciatica and/or pudendal/anogenital; pain; infertility | Bladder | Laparoscopic bladder resection with concomitant radical excision of DIE | 1; 6; 12 |
| 17 | Abesadze, et al. (31) | 2020 | Germany | Cohort | 54 | 35 ± 7 | Not mentioned | Dysmenorrhea, dysuria, dyschezie, dyspareunia, chronic pelvic pain, cyclical pelvic pain, infertility | Posterior compartment of the peritoneum | Complete excision | > 60 |
| 18 | Sun, et al. (32) | 2020 | China | Cohort | 59 | 31.8 ± 3.6 | 21.4 ± 2.3 | Infertility dysmenorrhea; chronic pelvic pain | Ovaries | Laparoscopic excision | 60; 72 |
| 19 | Nirgianakis, et al. (33) | 2020 | Switzerland | Cohort | 54 | 30.1 ± 5.0 | 23 | Infertility; dysuria or urinary urgency; dyschezia; deep dyspareunia; dysmenorrhea or pelvic pain | Rectovaginal septum | Laparoscopic segmental bowel resection | 36 |
| 20 | Ceccaroni, et al. (34) | 2019 | Italy | Cohort | 160 | 36.1 | 22.1 | Dysmenorrhea, dysuria, dyspareunia, and dyschezia | Ureteral, parametrial, and bowel | Laparoscopic ureteroneocystostomy | 1 -6 - 12 |
| 21 | Zheng, et al. (35) | 2019 | China | Cohort | 11 | 35 (range: 20 - 49) | 20.9 (range: 16.2 - 27.9) | Infertility, dysmenorrhea, dyspareunia ,rectal bleeding, tenesmus pelvic pain, dyschezia , micturition, intermenstrual bleeding | Bowel | Laparoscopic surgery | 23.2 |
| 22 | Shaltout, et al. (36) | 2019 | Egypt | RCT | 200 | Drainage only: 28.2 ± 4.1; cystectomy only: 26.6 ± 4.4; drainage & laparoscopy: 27.5 ± 3.7; cystectomy & laparoscopy: 27.9 ± 4.1 | Drainage only: 25.5 ± 1.3; cystectomy only: 25.3 ± 1.4; drainage & laparoscopy: 25.4 ± 1.3; cystectomy & laparoscopy: 25.3 ± 1.2 | Infertility; pelvic pain or pelvic mass unilateral & unilocular endometrioma | Ovaries | Laparoscopic approaches | 24 |
| 23 | Roman, et al. (37) | 2019 | France | RCT | 55 (Excision :27, Colorectal resection: 28 ) | Excision :30 (27 - 36) Colorectal resection: 28 (27 - 33) | NR | Constipation, frequent bowel movements, anal incontinence, dysuria, bladder atony | Bowel | Excision or Colorectal resection | 24 - 60 |
| 24 | Hernandez Gutierrez, et al. (9) | 2019 | Spain | Cohort | 143 | I: Segmental resection: 36.3 ± 5.6; II: Discoid resection: 34.9 ± 6.8; III: Nodule shaving: 36.6 ± 5.8 | Segmental resection: 21.8 ± 0.7; discoid resection: 21.05 ±1.2; nodule shaving: 21.6 ± 0.9 | Digestive symptoms | Ileum, cecum, appendix | Segmental resection; discoid resection; nodule shaving | 46.4 ± 0.5 months for the group I, 42.2 ± 1.6 months for the group II, 39.7 ± 1.8 months for the group III |
| 25 | Roman, et al. (38) | 2018 | France | RCT | 36 | 28 (range: 23 - 39) | 23.9 (range: 17.3 - 33.1) | Dysmenorrhea, dyspareunia, chronic intermenstrual pelvic pain, digestive symptoms, urinary symptoms, infertility | Rectaum | Conservative rectal surgery over segmental resection | 50 - 79 |
| 26 | Saavalainen, et al. (39) | 2016 | Finland | Cohort | 53 | 35.0 ± 4.4 | 23.1 ±3.7 | Dysmenorrhea, dysuria, pollakisuria, and/or hematuria, dyschezia and/or hematemesis, dyspareunia | Urinary tract | Laparoscopic surgery | 120 |
| 27 | Roman, et al. (40) | 2016 | France | Cohort | 71 (Shaving:46, colorectal resection:25) | Shaving: 41.5 (28 - 54), colorectal resection: 41 (31 - 54) | Not mentioned | Digestive symptoms | Rectum | Shaving; colorectal resection | Shaving: 72 (60 - 120), colorectal resection : 103 (60 - 118) |
| 28 | Roman, et al. (41) | 2016 | France | Cohort | 15 | Not mentioned | Not mentioned | Deep dyspareunia, severe constipation, feeling of incomplete rectal evacuation | Bowel | Colorectal resection (shaving, disc excision) | 12 - 36 |
| 29 | Afors, et al. (42) | 2016 | France | Cohort | 92 | Shaving: 32 ± 5.4; discoid: 29.47 ± 5.7; segmental resection: 31.12 ± 4.5 | Shaving:26.4 ± 3.4; discoid: 24.1 ± 5.2; segmental resection:27.3 ± 4.2 | Dysmenorrhea;dyspareunia; dyschezia; infertility | Bowel | Shaving, discoid; segmental resection | 3 & 24 |
| 30 | Cao, et al. (43) | 2015 | China | Cohort | 93 | 34.99 ± 7.15 | Not mentioned | Pelvic pain, bowel symptoms, dysmenorrhea, infertility | Cervical stump, vaginal stump, pelvic sidewall, bladder, ureter, rectum, cul-de-sac, rectovaginal septum, posterior fornix, uterosacral ligaments | Laparoscopic complete excision (n = 55), incomplete surgeryof DIE (n = 38) | 24 |
| 31 | Collinet, et al. (44) | 2014 | French | Cohort | 164 | 34.1 ± 7.3 | 24.4 ± 8.2 | Dysmenorrhea, chronic pelvic pain, dyspareunia, menometrorrhagia , urinary functional signs , digestive functional signs, Infertility | Rectum, bladder, ureter, uterosacral ligaments | Robot-assisted laparoscopy | 10.2 |
| 32 | Uccella, et al. (45) | 2014 | Italy | Cohort | 109 | 35 (20 - 54) | 21.5; (range: 16.3 - 31.6) | Dysmenorrhea; pelvic pain; dyspareunia; dyschezia; lower back pain; urinary symptoms; hematuria | Ureter | Laparoscopic ureterolysis | 15 - 109 |
| 33 | Ruffo, et al. (11) | 2014 | Italy | Cohort | 774 | 27.5 (22 - 51) | 23.7 (18.5 - 31.5) | Dyspareunia; constipation; pelvic pain; diarrhea | Bowel | Laparoscopic bowel resections | 54 |
| 34 | Nirgianaki, et al. (46) | 2014 | Switzerland | Cohort | 81 | 33 (24 - 49) | 22 (16 - 32) | Infertility; dysuria or urinary urgency; dyschezia; deep dyspareunia; dysmenorrhea or pelvic pain | Bowel | Laparoscopic segmental bowel resection | 120 |
| 35 | Nezhat, et al. (47) | 2014 | USA | Cohort | 25 | 37.7 (range: 25 - 60) | Not mentioned | Chest complaint; Shoulder pain; catamenial pneumothorax; hemoptysis | Thoracic and abdominopelvic | Combined video-assisted thoracoscopic surgery and traditional laparoscopy | 9; 12 |
| 36 | Mangler, et al. (48) | 2014 | Germany | Cohort | 71 | Median: 33.35 (range: 24 - 39) | Median: 23 (range: 17 - 31) | Dysmenorrhea; hypermenorrhea dyspareunia; chronic pelvic pain defecating symptoms; dyschezia; hematochezia; cyclic rectal bleeding; diarrhea and constipation; dysuria; back pain Infertility | Bowel | Surgical nerve-sparing approach | Median:63.9 (range: 6 - 98) |
| 37 | Neme, et al. (49) | 2013 | Brazil | Cohort | 10 | Median :37 (range: 29 - 48) | Median : 23.5 (range: 20 - 26) | Pelvic pain,Infertility,dysmenorrhea, dyspareunia, dyschezia,intestinal cramping, diarrhea, & constipation | Colorectal | Robotic-assistedlaparoscopic colorectal resection | 12 |
| 38 | Schonman, et al. (50) | 2013 | Israel | Cohort | 7 | 34.3 ± 5.5 | Not mentioned | Dysmenorrhea, dyspareunia, flank pain (urinary symptoms), | Ureter | Ureteral reimplantation | 42.3 - 20.0 |
| 39 | Mabrouk, et al. (51) | 2012 | Italy | Cohort | 47 | Median: 34 (range : 25 - 39) | Median: 21 (range: 17 - 29) | Infertility, tenesmus, abdominal distension, rectal bleeding, constipation, diarrhoea, nausea and vomiting, pain on defecation, dysparaeunia, chronic pelvic pain, dysmenorrhea | Colorectal | Laparoscopic segmental resection | 18 |
| 40 | Koh, et al. (52) | 2012 | Australia | Cohort | 91 | Mean: 35 (range: 22 - 46) | 24.1 | Dysmenorrhea,menorrhagia, dyspareunia,infertility, pelvic/low-back pain, dyschezia, urgency/diarrhea/tenesmus, rectal bleeding | Rectal | Disc resection, Segmental resections | 120 |
| 41 | Jelenc, et al. (53) | 2012 | Slovenia | Cohort | 52 | Mean: 34.4 (range: 22 - 62) | Not mentioned | Dysmenorrhea, dyspareunia,chronic pelvic pain, infertility | Colorectal | Laparoscopic disk resection | 84 |
Abbreviations: BMI, Body Mass Index; DIE, deep infiltrating endometriosis.
3.5. Quality Assessment
The studies were evaluated using the Mixed Methods Appraisal Tool (MMAT), version 2018. This tool is specifically designed to assess the quality of empirical studies, including primary research based on experiments, observations, or simulations. Its primary purpose is to provide a systematic approach for appraising the quality of these studies (54, 55). The tool comprises five items for each category, with responses marked as "yes," "no," or "not known." In the scoring system, a "yes" answer is scored as 1, while all other responses are scored as 0. A higher score indicates higher quality. For the final quality assessment, scores above half (more than 50%) were considered high quality (Table 2).
| Selected Studies | Appraisal Quality | Quantitative Non-randomized Criteria | ||||
|---|---|---|---|---|---|---|
| Are the Participants Representative of the Target Population? | Are Measurements Appropriate Regarding Both the Outcome and Intervention (or Exposure)? | Are There Complete Outcome Data? | Are the Confounders Accounted for in the Design and Analysis? | During the Study Period, Is the Intervention Administered (or Exposure Occurred) as Intended? | ||
| Missori, et al. (15) | H | Y | Y | Y | Y | Y |
| Han, et al. (16) | H | Y | Y | Y | Y | Y |
| Zhang, et al. (17) | H | Y | Y | Y | Y | Y |
| Yang, et al. (18) | H | Y | N | Y | C | Y |
| Leborne, et al. (19) | H | Y | Y | Y | Y | Y |
| Zhang et al. (20) | H | Y | Y | Y | C | Y |
| Kim et al. (21) | H | Y | Y | Y | Y | Y |
| Roman et al. (22) | H | Y | Y | Y | C | Y |
| Ceccaroni, et al. (23) | H | Y | Y | Y | Y | Y |
| Sarbazi, et al. (24) | H | Y | Y | Y | Y | Y |
| Yela, et al. (25) | H | Y | Y | Y | Y | Y |
| Vidal, et al. (26) | H | Y | Y | Y | Y | Y |
| Parra, et al. (27) | H | Y | Y | Y | Y | Y |
| Jayot, et al. (28) | H | Y | Y | Y | Y | Y |
| Abesadze, et al. (29) | H | Y | Y | Y | C | Y |
| Ceccaroni, et al. (30) | H | Y | Y | Y | Y | Y |
| Abesadze, et al. (31) | H | Y | Y | Y | C | Y |
| Sun, et al. (32) | H | Y | Y | Y | C | Y |
| Nirgianakis, et al. (33) | H | Y | Y | N | Y | Y |
| Ceccaroni, et al. (34) | H | Y | Y | Y | Y | Y |
| Zheng, et al. (35) | H | Y | C | Y | C | Y |
| Shaltout, et al. (36) | H | Y | Y | Y | C | Y |
| Roman, et al. (37) | H | Y | Y | Y | C | Y |
| Hernandez Gutierrez, et al. (9) | H | Y | Y | Y | Y | Y |
| Roman, et al. (38) | H | Y | Y | Y | Y | Y |
| Saavalainen, et al. (39) | H | Y | Y | Y | Y | Y |
| Roman, et al. (40) | H | Y | Y | Y | Y | Y |
| Roman, at al. (41) | H | Y | Y | Y | C | Y |
| Afors, et al. (42) | H | Y | Y | Y | Y | Y |
| Cao, et al. (43) | H | Y | Y | Y | N | Y |
| Collinet, et al. (44) | H | Y | Y | Y | Y | Y |
| Uccella, et al. (45) | H | Y | Y | Y | Y | Y |
| Ruffo, et al. (11) | H | Y | Y | Y | Y | Y |
| Nirgianaki, et al. (46) | H | Y | Y | Y | C | Y |
| Nezhat, et al. (47) | H | Y | Y | Y | N | C |
| Mangler, et al. (48) | H | Y | Y | Y | Y | C |
| Neme, et al. (49) | H | Y | Y | Y | C | C |
| Schonman, et al. (50) | H | Y | Y | C | N | Y |
| Mabrouk, et al. (51) | H | Y | Y | Y | Y | Y |
| Koh, et al. (52) | H | Y | Y | Y | Y | Y |
| Jelenc, et al. (53) | H | Y | Y | Y | C | Y |
a Scoring: Y, yes, N, no, C, can’t tell, H, high.
3.6. Data Extraction
Two researchers independently conducted the study selection and validity assessment, resolving any discrepancies by consulting a third researcher. The studies extracted information on various parameters, including author, year, study design, country, age, number of participants, Body Mass Index (BMI), symptoms, location of endometriosis, surgical techniques, recurrence rate, post-surgical pregnancy rate, and follow-up duration (Table 1).
3.7. Data Synthesis
A comprehensive analysis was conducted by performing a quantitative synthesis using STATA software version 17. The random-effects model was employed for the meta-analysis due to the inclusion of studies from diverse populations. This model accounts for both within-study and between-study variances, thereby ensuring a thorough analysis (56). The Q Cochrane statistic was used to evaluate heterogeneity, while the I² index was utilized to quantify the extent of heterogeneity. Heterogeneity was interpreted as (i) mild if the I² value is below 25%, (ii) moderate if the I² value ranges from 25% to 50%, (iii) severe if the I² value falls between 50% and 75%, and (iv) highly severe if the I² value exceeds 75% (57).
The key measures selected for this study were the prevalence of endometriosis and the pregnancy rate after surgery. To determine the overall prevalence, numerical findings for these conditions were combined, and a pooled prevalence was calculated. Additionally, a 95% confidence interval (CI) was provided to indicate the range of possible prevalence values.
To evaluate moderator effects, subgroup analysis, or meta-regression, an assessment was performed considering the number of studies in each group. In cases where the number of studies in a particular group was fewer than four, meta-regression was employed. Publication bias was assessed using a funnel plot, as well as Begg's Test and Egger's Test (58). Sensitivity analysis was conducted using the Jackknife method (59).
4. Results
4.1. Study Screening & Selection Process
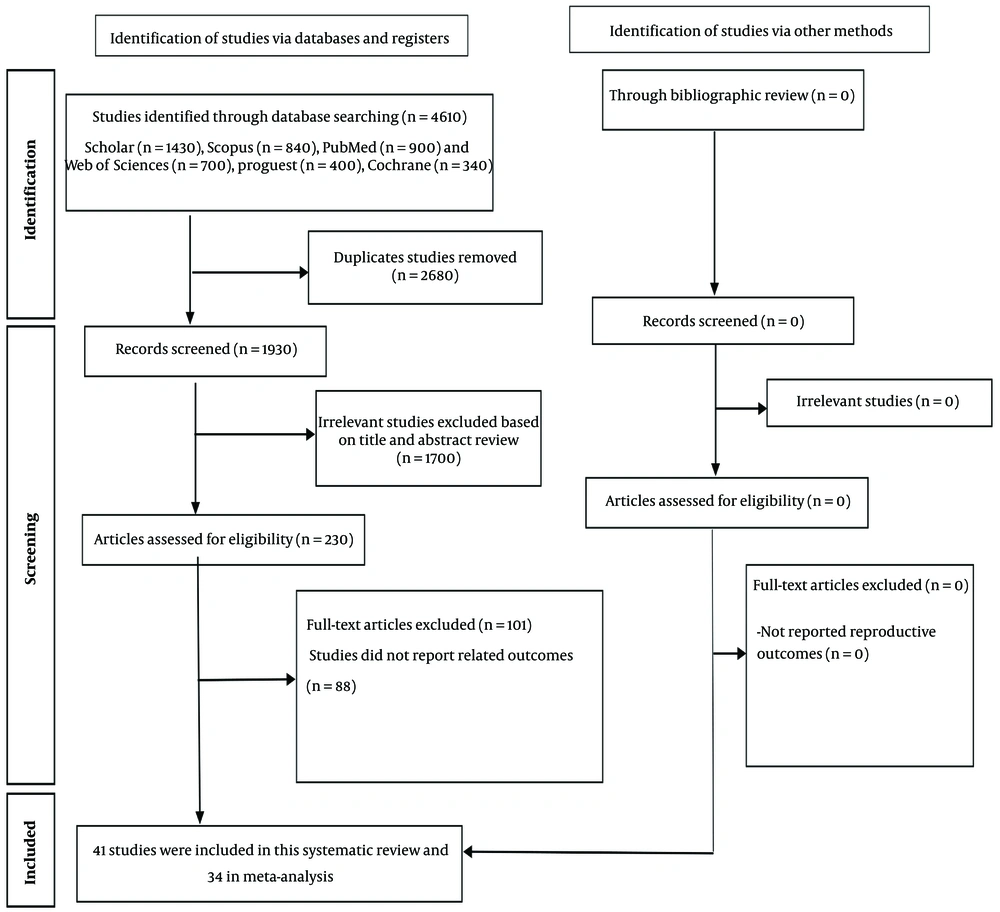
The initial search yielded 4,610 results. Two authors independently evaluated the eligibility of these studies, with disagreements resolved through consensus by consulting a third author. In the first stage, 2,680 irrelevant or duplicate articles were excluded. After reviewing the titles and abstracts of the remaining articles, additional papers were excluded. Ultimately, a total of 41 eligible studies were systematically reviewed, and 34 studies met the criteria for inclusion in the meta-analysis (Figure 1). Key findings from the included studies are summarized in Table 1.
4.2. Studies Characteristics
Thirty-four papers, comprising 6,514 individuals from 14 countries (e.g., Australia, Brazil, China, Egypt, France, Germany, Iran, Israel, Italy, Korea, Spain, Switzerland, Slovenia, and the USA), were included in the analysis regarding endometriosis recurrence. The two countries with the highest number of eligible studies were France (n = 7) and Italy (n = 5). The smallest sample size was 7 participants, and the largest sample size was 1,332.
The mean age of participants was 33.92 years, with a range of 27.5 to 41 years (reported in 34 studies). The mean BMI of participants was 23.18 kg/m², with a range of 20.9 to 26.9 kg/m² (reported in 22 studies). The mean follow-up duration was 43.21 months, ranging from 10 to 120 months (reported in 35 studies). The most frequently reported endometriosis lesion sites were bowel (n = 9), rectal (n = 8), and DIE (n = 7).
4.3. Endometriosis Recurrence
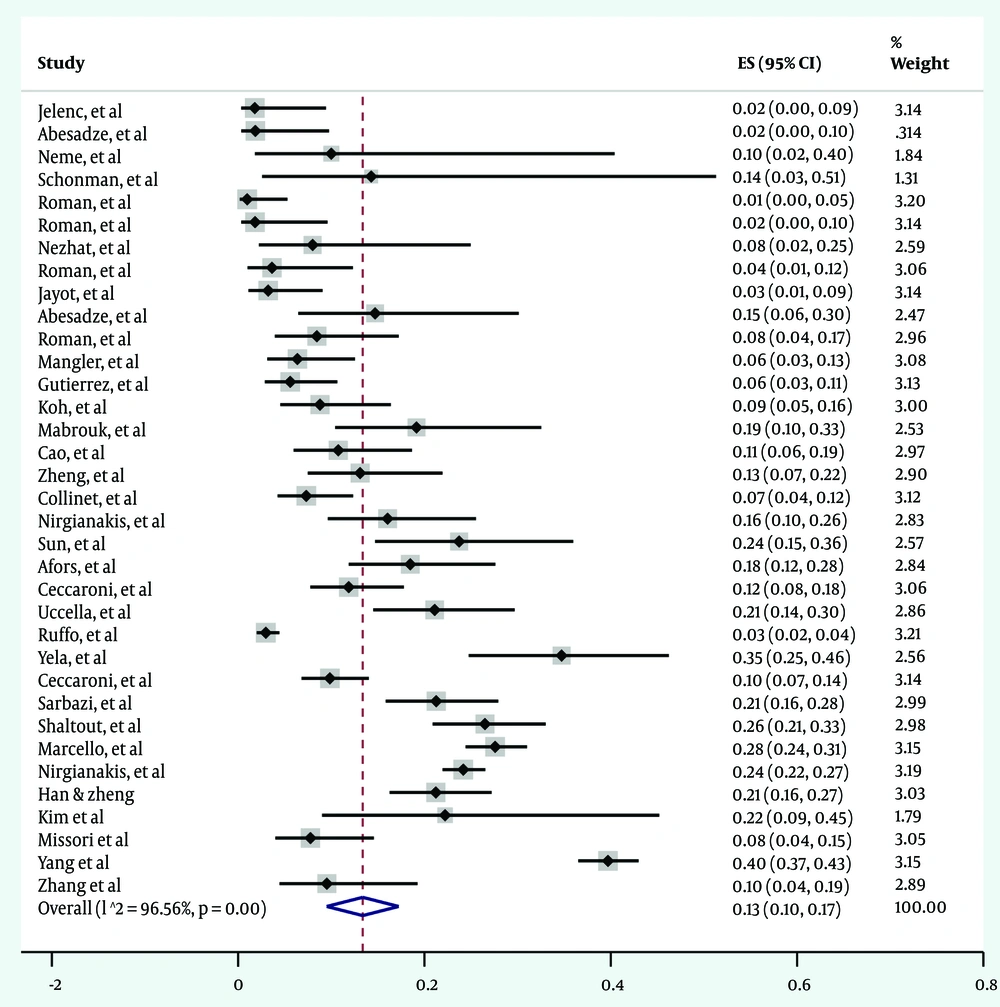
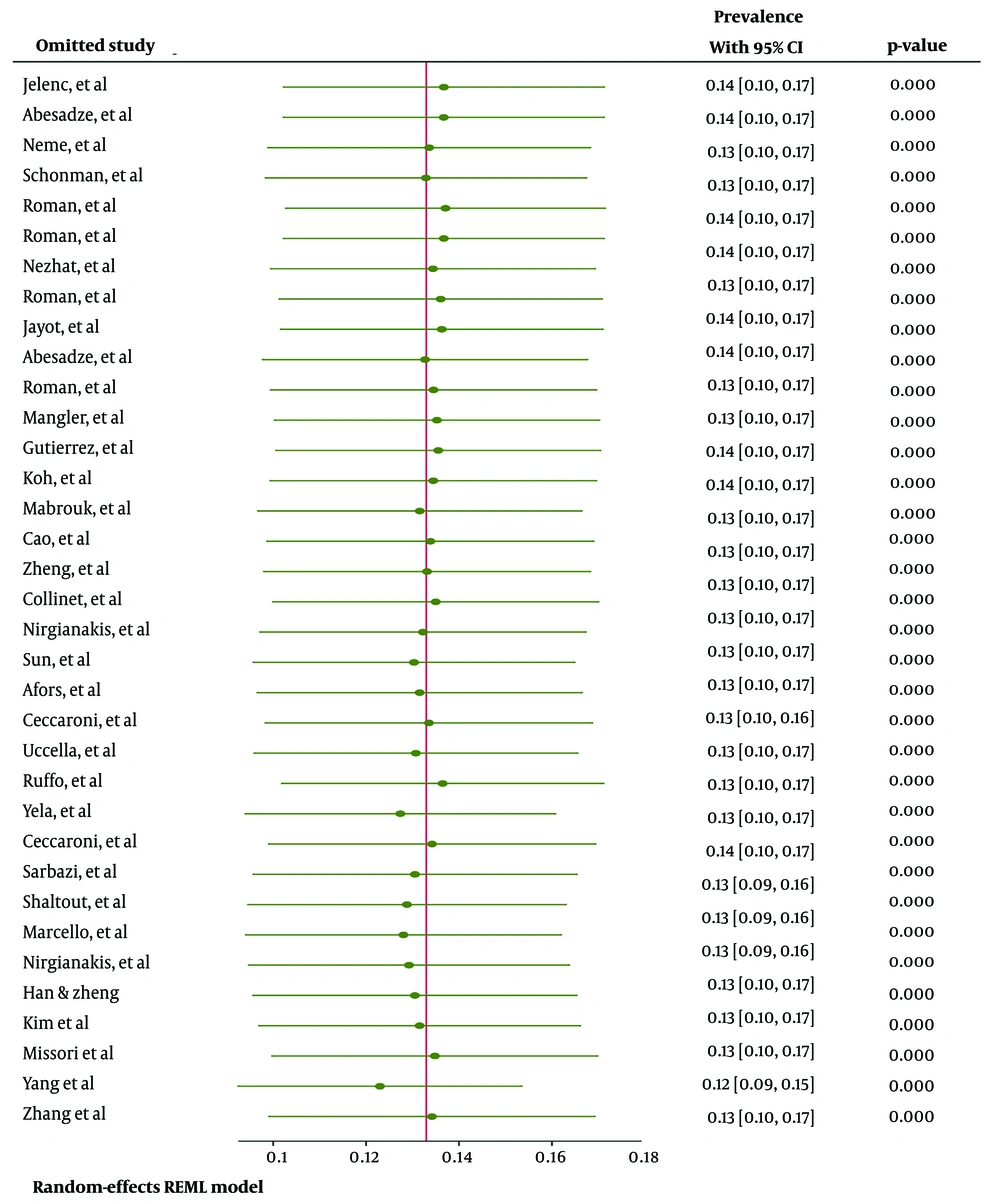
The pooled estimated prevalence of endometriosis recurrence was 13% [95% CI: 11 - 17%, I²: 96.5%, Tau²: 0.01, Observations: 35]. Figure 2 presents the forest plot illustrating the pooled prevalence of endometriosis recurrence across the included studies.
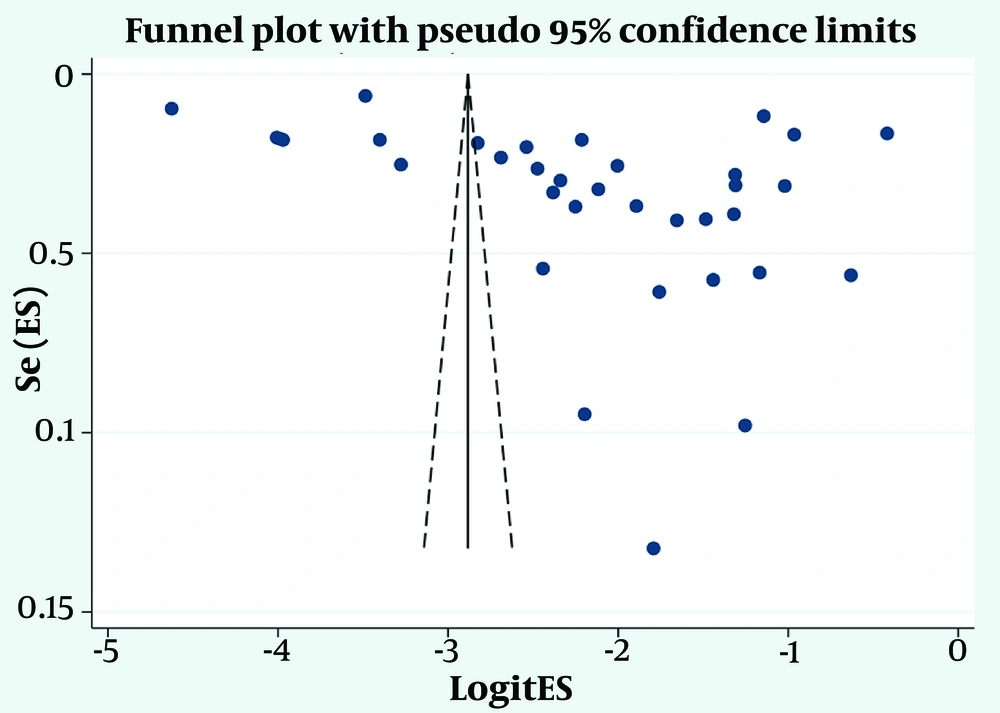
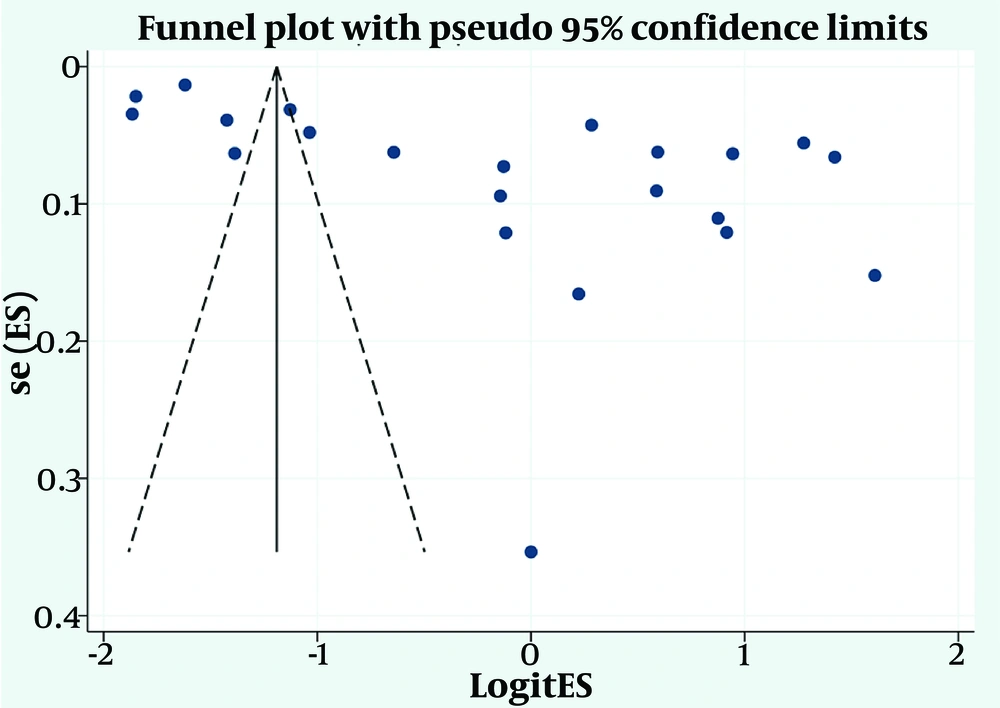
Based on Egger’s test (P = 0.056) and the asymmetric funnel plot (Figure 3), the likelihood of publication bias appeared probable. To further evaluate this, the fill-and-trim method was applied. Using this method, no additional studies were imputed, and the probability of publication bias was ultimately ruled out.
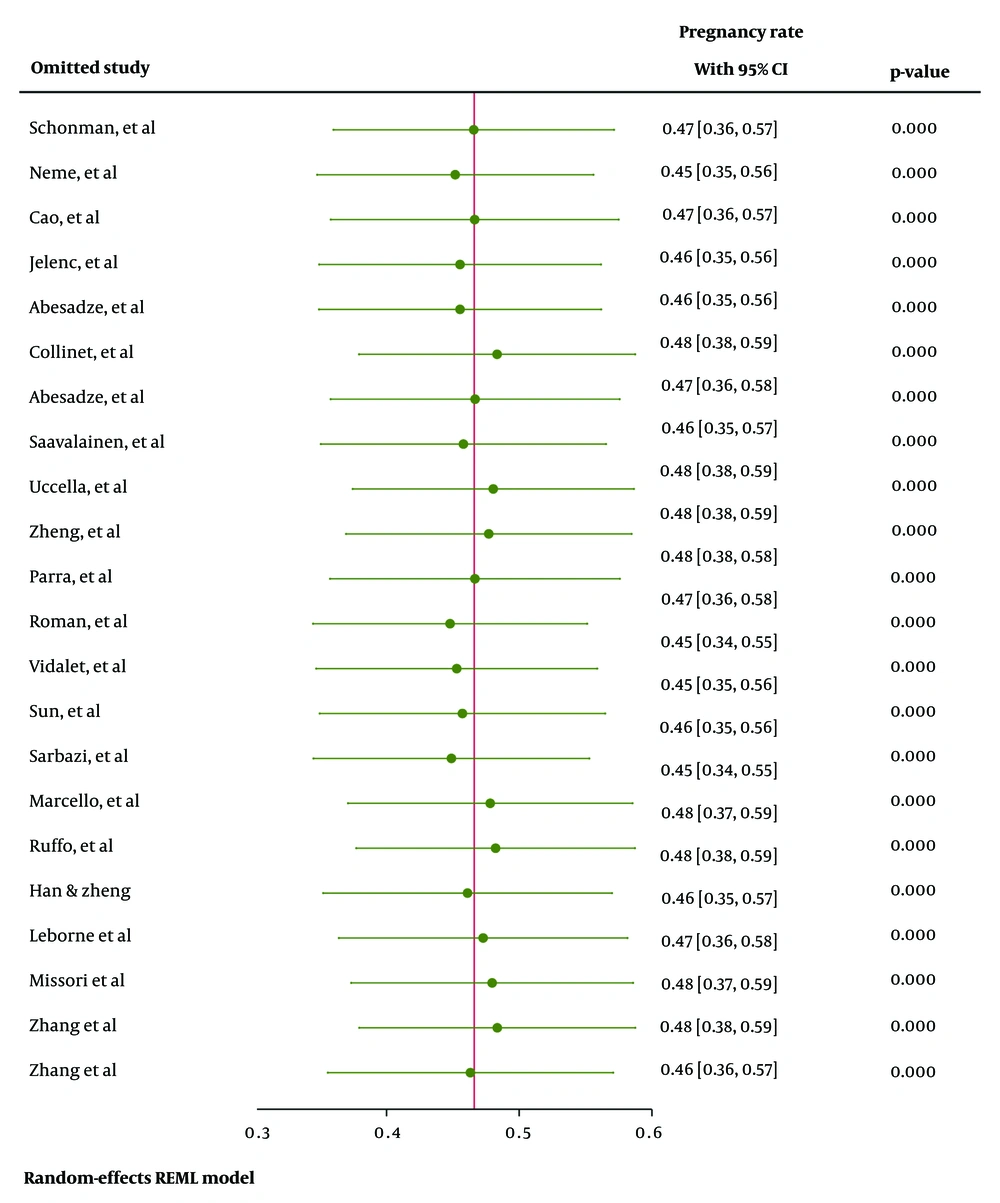
Additionally, sensitivity analysis (Figure 4) indicated that the pooled effect size was not influenced by the effect of any single study.
4.4. Pregnancy Rate After Surgery
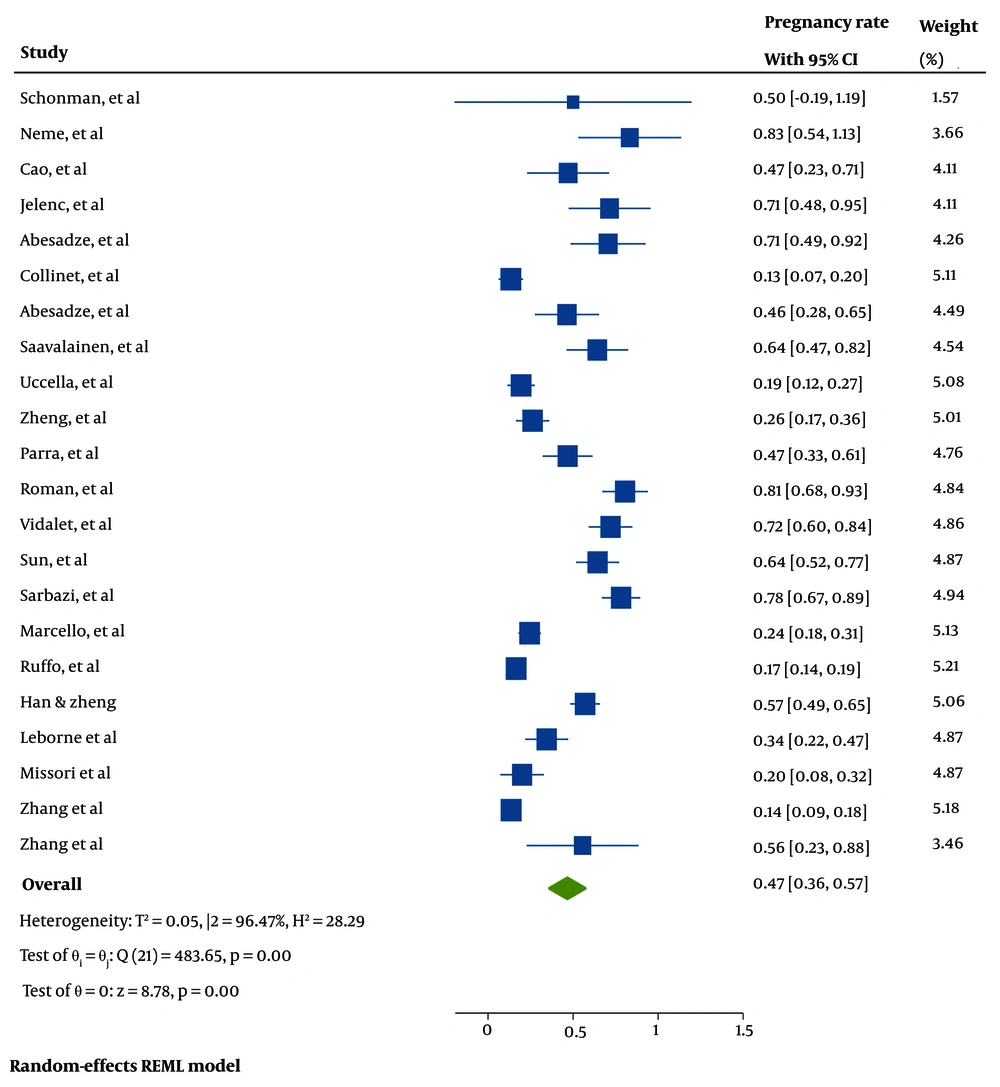
Twenty-two papers, comprising 2,039 individuals with infertility from nine countries (e.g., Brazil, China, Finland, France, Germany, Israel, Italy, Iran, and Slovenia), were included in the analysis of pregnancy rates after surgery for endometriosis. The highest number of eligible studies were from China (n = 6). The smallest sample size was 7 participants, and the largest was 774.
The mean age of participants was 33.44 years, ranging from 27.5 to 37 years (reported in 22 studies). The mean BMI of participants was 23.41 kg/m², ranging from 20.9 to 25.7 kg/m² (reported in 15 studies). The mean follow-up duration was 44.30 months, ranging from 10 to 120 months (reported in 22 studies). The most frequently reported endometriosis lesion sites were DIE (n = 8 studies).
The pooled estimated pregnancy rate after surgery for endometriosis was 47% [95% CI: 36 - 57%, I²: 96.47%, Tau²: 0.05]. Figure 5 presents the forest plot illustrating the pooled prevalence of pregnancy rates after surgery for endometriosis across the included studies.
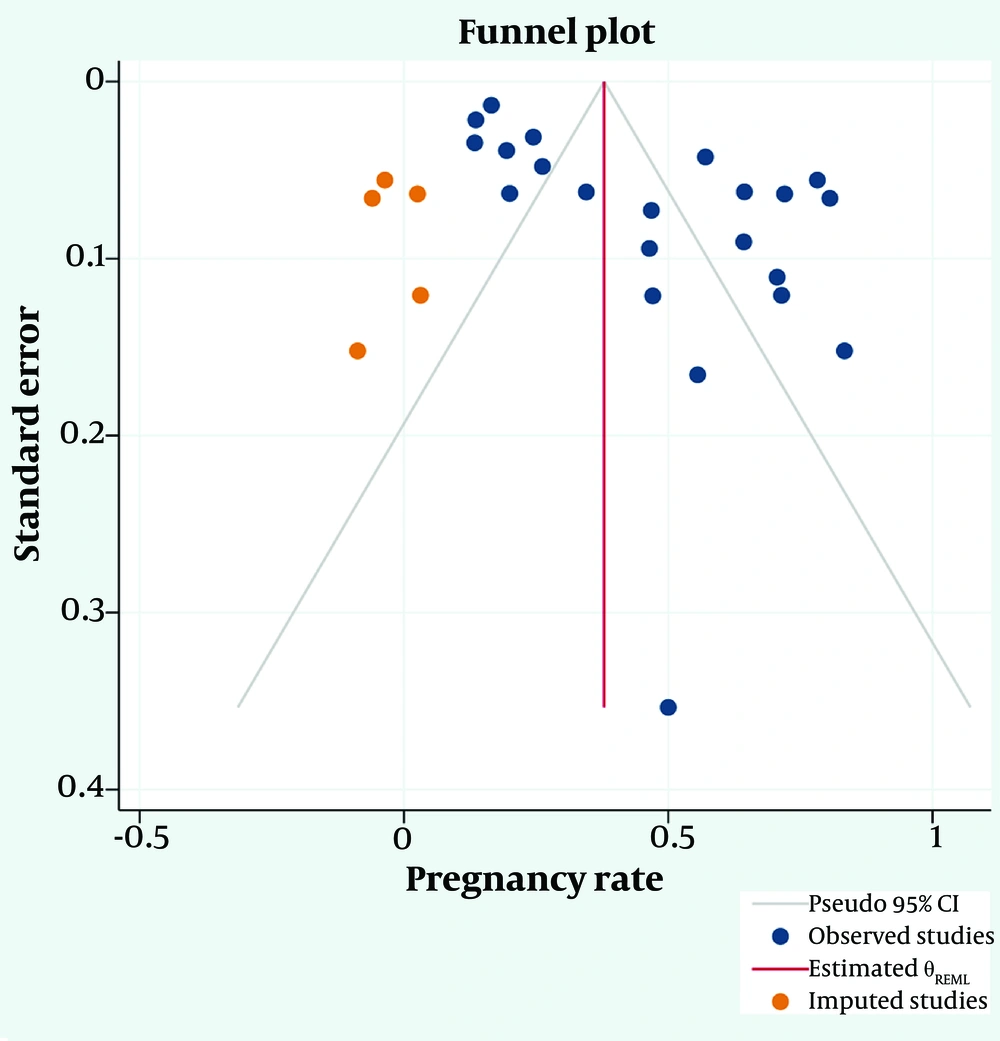
Based on Egger’s test (P < 0.001) and the asymmetric funnel plot (Figure 6), publication bias appears to be probable.
Probable publication bias was addressed using the fill-and-trim method. In this process, five studies were imputed, resulting in a corrected pooled prevalence of the pregnancy rate after surgery for endometriosis of 37.9% (95% CI: 26.8 - 48.9%). The funnel plot after trimming is presented in Figure 7.
Sensitivity analysis (Figure 8) demonstrated that the pooled effect size was not influenced by the effect of any single study. Based on meta-regression (Table 3), none of the examined variables significantly predicted the prevalence of the pregnancy rate after surgery for endometriosis.
The pooled estimated prevalence of preoperative dysmenorrhea was 78% (22 papers, 95% CI: 64 - 92%, I²: 99.40%, Tau²: 0.11), while postoperative dysmenorrhea was 24% (8 papers, 95% CI: 14 - 34%, I²: 97.51%, Tau²: 0.02).
| Variables | Endometriosis Recurrence | Pregnancy Rate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Coeff. | S.E. | P | I2 res. (%) | R2 (%) | Tau2 | No. of Studies | Coeff. | S.E. | P | I2 res. (%) | R2 (%) | Tau2 | |
| Country | 35 | 0.002 | 0.005 | 0.61 | 95.44 | 0 | 0.009 | 22 | 0 .02 | 0.02 | 0.19 | 95.94 | 4.01 | 0.05 |
| Study design | 35 | 0.02 | 0.03 | 0.53 | 95.51 | 0 | 0.009 | 22 | 0.15 | 0.11 | 0.17 | 96.34 | 5.42 | 0.05 |
| Mean age | 34 | 0.002 | 0.005 | 0.78 | 94.25 | 0 | 0.009 | 22 | -0.004 | 0.02 | 0.84 | 95.86 | 0 | 0.06 |
| Mean BMI | 22 | 0.02 | 0.01 | 0.18 | 91.98 | 3.13 | 0.006 | 15 | 0.05 | 0.05 | 0.38 | 97.01 | 0 | 0.06 |
| Fallow up time | 35 | -0.001 | 0.0006 | 0.36 | 95.39 | 0.13 | 0.009 | 22 | 0.001 | 0.002 | 0.45 | 96.31 | 0 | 0.05 |
| Endometriosis lesion site | 35 | 0.008 | 0.007 | 0.21 | 95.31 | 1.97 | 0.009 | 22 | 0.02 | 0.02 | 0.49 | 96.14 | 0 | 0.05 |
Abbreviation: BMI, Body Mass Index.
The pooled estimated prevalence of preoperative chronic pelvic pain was 50% (17 papers, 95% CI: 35 - 64%, I²: 98.98%, Tau²: 0.09), and postoperative chronic pelvic pain was 31% (7 papers, 95% CI: 15 - 37%, I²: 96.21%, Tau²: 0.04).
The pooled estimated prevalence of preoperative dyspareunia was 56% (20 papers, 95% CI: 42 - 71%, I²: 98.91%, Tau²: 0.10), while postoperative dyspareunia was 22% (6 papers, 95% CI: 5 - 39%, I²: 96.55%, Tau²: 0.04).
The pooled estimated prevalence of preoperative dyschezia was 44% (15 papers, 95% CI: 32 - 57%, I²: 98.19%, Tau²: 0.06), and postoperative dyschezia was 21% (5 papers, 95% CI: 5 - 36%, I²: 95.97%, Tau²: 0.03).
5. Discussion
In this systematic review and meta-analysis, we identified 41 studies evaluating pregnancy and recurrence rates after surgical treatments in women with DIE. The results demonstrated that the prevalence of endometriosis recurrence was 13%, while the pregnancy rate after surgery was estimated at 47%. Additionally, we concluded that the postoperative prevalence of dysmenorrhea was 24%, chronic pelvic pain 31%, dyspareunia 22%, and dyschezia 21%. Compared to preoperative rates, the prevalence of these symptoms had decreased.
A study investigating the efficacy of laparoscopic ureteroneocystostomy in patients with DIE involving the ureter, parametrial region, and bowel showed that among 60 patients with DIE, the recurrence rate was reported as 1.2% after six months of follow-up. This study concluded that laparoscopic partial cystectomy for DIE is the gold standard treatment due to its low recurrence rate (30). Ferrero et al. (2020) examined the risk of recurrence after segmental resection for rectosigmoid endometriosis. After a five-year follow-up, imaging detected rectosigmoid endometriosis recurrence in five patients. Surgical and histological diagnoses confirmed recurrence in six out of seven patients (60).
Hernandez Gutierrez et al. (2019) compared postoperative complications and recurrence rates among three surgical techniques: Segmental resection, discoid excision, and nodule shaving. Their findings revealed that segmental resection had a significantly higher incidence of severe postoperative complications compared to discoid excision or the shaving technique (23.5% versus 5% versus 0%, respectively). However, over an extended follow-up period, the shaving group exhibited a higher recurrence rate (12.7%) compared to the discoid group (5%) and the segmental resection group (1.3%) (9).
Cao et al. (2015) evaluated the efficacy and safety of complete versus incomplete excision of DIE. Their results indicated that recurrence rates were significantly higher in the incomplete excision group (29.4% vs. 3.9%) (43). A comprehensive analysis and meta-analysis investigating recurrence following surgical treatment for colorectal endometriosis found that the risk of recurrence was higher after rectal shaving compared to segmental resection and disc excision in cases with confirmed histological recurrence. However, no significant difference was observed between the recurrence rates of disc excision and segmental resection (61).
Another review study highlighted that incomplete removal of endometriosis is a major contributing factor to recurrence, as documented in the literature. The extent of lesion excision significantly influences recurrent symptoms, especially based on the type of hysterectomy performed. Notably, no studies have specifically compared recurrence rates of endometriosis following standard hysterectomy with robotic-assisted hysterectomy (62).
In the present study, we reported an overall recurrence rate of DIE after surgical procedures (regardless of the type of surgery and the location of endometriosis) as 13%. Several risk factors appear to influence the recurrence rate of endometriosis. These factors include young women affected by the condition who desire pregnancy but decline hormonal treatments following surgery; the location of endometriosis, particularly when it affects the bladder and uterus; women who are obese or overweight; the primary surgical approach employed; and incomplete removal of lesions (7, 12). Additionally, the presence of microscopic satellite lesions adjacent to the main lesion, which may remain undetected during surgery, can contribute to an increased incidence of recurrence (63, 64).
Regarding symptoms associated with DIE (dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia), our findings showed that surgery improved these symptoms compared to the preoperative condition. When assessing pain in women with endometriosis during treatment trials, three factors are crucial: The use of a valid pain scale, time-dependent assessment, and consideration of placebo or sham surgery effects (61).
Jayot et al. (2020) investigated various factors in a group of patients who underwent discoid resection. These factors included the conversion rate to segmental resection, the necessity for double discoid resection, and the rates of complications and recurrence. Their findings revealed no significant differences in complication rates or voiding dysfunction between double and single discoid resection groups (28).
In a 2006 analysis, the crude pain recurrence rate in women with endometriosis undergoing first-line conservative laparoscopic surgery was reported to be 21%, and the crude disease relapse rate was 9% (5). Many studies consider recurrence as the reappearance of pain; however, this definition has limitations due to the subjective nature of pain evaluation (40). Although surgical excision of endometriosis improves pain and enhances fertility, recurrence can exacerbate pain and reduce fertility, negatively impacting quality of life and increasing personal and social costs.
Surgical techniques may also influence symptom recurrence. For example, a study revealed that individuals who underwent hysterectomy with ovarian conservation for endometriosis had a significantly higher risk of recurrent pain and reoperation compared to those who underwent oophorectomy. Specifically, the former group had a 6.1-fold greater risk of recurrent pain and an 8.1-fold greater risk of reoperation (65).
Among the strengths of this study are the following: Separating the types of endometriosis and the surgical techniques used for each type of lesion, examining other factors affecting endometriosis recurrence rates, such as BMI, and evaluating factors influencing the effectiveness of surgical methods, in addition to recurrence rates, such as pregnancy rates (distinguishing between natural pregnancy and ART use). Moreover, the study assessed the recurrence of symptoms related to endometriosis, such as dysmenorrhea and dyspareunia.
One limitation of this study is the lack of separate recurrence rate estimates for each surgical approach or for each specific site of endometriosis. Future studies should address these issues in their analyses. Additionally, it is suggested to evaluate recurrence rates in cases where medical approaches are used post-surgery.
Although significant efforts were made to conduct a comprehensive and precise search within scientific databases, there remains a possibility that some relevant studies were overlooked due to constraints such as limited resource accessibility, the selection of specific search terms, or the restricted publication of certain articles. Furthermore, while study quality was assessed using established and validated tools, the potential for human error in scoring or interpreting evaluation criteria cannot be entirely excluded. These limitations may affect the outcomes despite diligent attempts to minimize biases.
5.1. Conclusions
Two critical considerations in selecting the treatment approach for women with DIE are the recurrence and pregnancy rates following treatment. Recurrence after DIE treatment has a significant negative impact on women's quality of life. Therefore, efforts should focus on improving their quality of life by selecting the most effective treatment approach.
In this study, the overall recurrence rate for DIE following various surgical approaches was reported to be approximately 13%, while the pregnancy rate was 47%. These findings provide valuable insights for choosing the best treatment method for women who are suitable candidates for surgery. However, due to the diversity of surgical methods used and the limited number of cases for each method, further studies with larger sample sizes and varied designs are needed. These future studies would enable more informed decisions in this field.