1. Background
Staphylococcus aureus is a highly hazardous bacterium. Although it is part of the human body's normal microflora, it has the potential to cause various infections, ranging from minor skin conditions to severe diseases such as osteomyelitis (1). The increasing prevalence of antibiotic-resistant bacteria, including community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), presents a significant global health threat (2-4).
The overuse and misuse of antibiotics have created a vicious cycle, whereby bacterial strains develop resistance, necessitating the use of stronger or additional antibiotics, which in turn accelerates the evolution of resistance (3-5). This defective cycle of antibiotic resistance has led to the emergence of increasingly virulent and difficult-to-treat strains of S. aureus, further complicating public health efforts. The critical nature of these infections has resulted in a rise in the use of anti-staphylococcal antibiotics, which exacerbates the resistance problem.
Addressing antibiotic-resistant S. aureus remains a significant health challenge. Recognizing this urgency, the World Health Organization (WHO) has designated S. aureus as a top priority for new drug development. This underscores the pressing need for innovative treatments, such as photodynamic therapy (PDT), to combat this growing threat (1, 3-5).
Photodynamic therapy is one of the recommended antimicrobial treatments (5-10). While PDT has primarily been utilized as a cancer treatment due to its effective antitumor properties, its potential as an antimicrobial therapy has received comparatively less attention (5, 6, 9, 10). In PDT, photosensitizer molecules play a crucial role in inducing targeted cell damage through photo-oxidative stress. These molecules absorb visible light of a specific wavelength and transfer the energy to molecular oxygen, generating reactive oxygen species (ROS). The ROS then induce photo-oxidative stress on organic molecules such as lipids and proteins in the bacterial envelope, ultimately leading to non-specific bacterial death with no possibility of resistance development (5, 6, 10). As the effectiveness of PDT is heavily dependent on the chemical structure of the photosensitizer molecules, empirical testing of both established and new formulations is essential. This study seeks to explore the effects of PDT on Staphylococcus aureus using two photosensitizer molecules: Phycocyanin and methylene blue. Phycocyanin is a blue pigment commonly employed in the food industry to enhance product aesthetics. Methylene blue (MB), initially developed as a textile dye, is also used as a surgical stain. Both components possess effective antioxidant properties and demonstrate potential as photosensitizers in PDT applications (11-13). However, no prior research has adequately compared the efficacy of these two mediators.
2. Objectives
This study aimed to compare the antibacterial effects of 660 nm laser therapy with phycocyanin and methylene blue on S. aureus colonies.
3. Methods
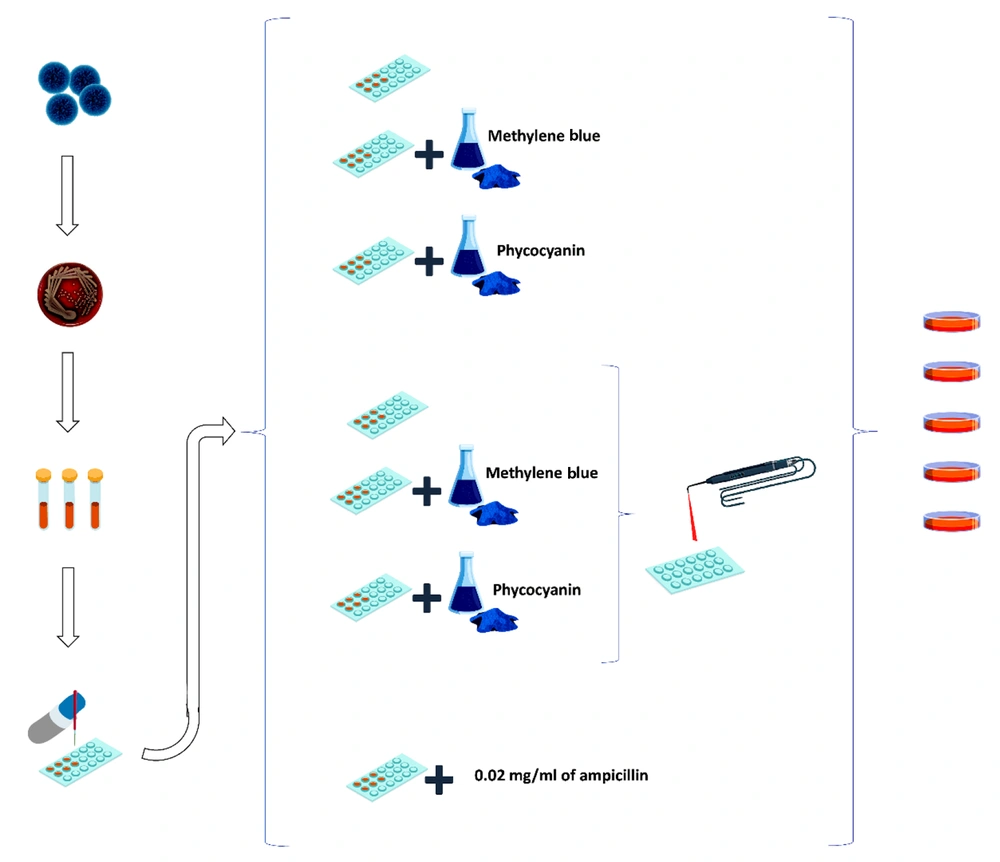
This study was an experimental in-vitro investigation. Figure 1 illustrates a schematic representation of the study methodology.
3.1. Sample Size
The articles by Gholibegloo et al. (14) and Afrasiabi et al. (13) reported colony reduction rates ranging from 18% to 91%. To detect changes in colony numbers from 1% to 111%, with an average reduction of 11%, a power of 81%, and an alpha error of 1% (to minimize the likelihood of a Type I error, ensuring that any statistically significant results are robust and less likely to occur by random chance), three samples were required for each subgroup.
The present study analyzed a total of seven main groups, four of which contained nine subgroups each. The remaining three groups had only one subgroup each. For each subgroup, the examinations were repeated three times, resulting in a total of 117 samples.
3.2. Staphylococcus aureus Microorganisms’ Culture
Lyophilized powder of S. aureus strain (ATCC433000, Pasteur Institute, Tehran, Iran) was reconstituted in nutrient broth (Catalog No. 105433, Merck, Germany) and incubated at 37 degrees Celsius (°C) for 24 hours. Following this, the culture was transferred to a blood agar medium (Catalog No. 110886, Merck, Germany) and incubated at 37°C for another 24 hours. Once the purity of the culture was confirmed, a suspension was prepared at a dilution of 0.5 McFarland (1.5 × 10⁸ colony-forming units/milliliter) (15).
3.3. Eligibility Criteria
Only S. aureus strains obtained from a certified source (ATCC433000 strain from the Pasteur Institute, Tehran, Iran) were used in this study. Samples with an optical density matching the 0.5 McFarland standard (1.5 × 10⁸ CFU/mL) were included to ensure consistent and comparable bacterial loads across all experimental groups. Samples exhibiting signs of contamination or impurity during the culturing process were excluded. Additionally, bacterial cultures that failed to reach the required 0.5 McFarland standard concentration or demonstrated irregular growth or inconsistency in colony-forming unit (CFU) counts during the pre-experimental phase were excluded from the experiments.
3.4. Preparation of Photosensitizer Concentration
A 2 milligrams per milliliter (mg/mL) concentration of phycocyanin (Phoenix Microalgae Co., Iran) and a 0.1 mg/mL concentration of MB (Catalog No. 159270, Merck, Germany) were initially prepared. The phycocyanin concentration was subsequently reduced to 0.005 mg/mL, and the MB concentration was diluted to 0.02 mg/mL using physiological serum. Both solutions were then serially diluted with 10 mL of Dimethyl sulfoxide (DMSO) (Catalog No. 1.02952, Merck, Germany), achieving final concentrations of 0.00001 mg/mL and 0.00005 mg/mL, respectively (16, 17).
3.5. The Examined Groups
In this study, seven main groups, divided into 39 subgroups based on different photosensitizer concentrations, were analyzed. The main groups were as follows:
(1) Staphylococcus aureus in the culture medium alone
(2) Staphylococcus aureus with MB at concentrations of 0.02, 0.01, 0.005, 0.002, 0.001, 0.0005, 0.0002, 0.0001, 0.00005 mg/mL, without laser irradiation
(3) Staphylococcus aureus with phycocyanin at concentrations of 0.005, 0.002, 0.001, 0.0005, 0.0002, 0.0001, 0.00005, 0.00002, 0.00001 mg/mL, without laser irradiation
(4) Staphylococcus aureus with laser irradiation only
(5) Staphylococcus aureus with laser irradiation and phycocyanin at concentrations of 0.005, 0.002, 0.001, 0.0005, 0.0002, 0.0001, 0.00005, 0.00002, 0.00001 mg/mL
(6) Staphylococcus aureus with laser irradiation and MB at concentrations of 0.02, 0.01, 0.005, 0.002, 0.001, 0.0005, 0.0002, 0.0001, 0.00005 mg/mL
(7) Staphylococcus aureus with 0.02 mg/mL of ampicillin (AMP) without laser irradiation (13, 14, 18).
3.6. Light Source and the Photodynamic Therapy Procedure
Ten microliters (λ) of S. aureus suspension and 10 λ of photosensitizer substances were pipetted into each well of a microplate using a sampler. In the laser-irradiated groups, each well was exposed to a 660-nm wavelength laser (Hamerz Rad Co., Iran) with a power output of 100 milliwatts and a beam diameter of 0.8 cm. The laser was applied continuously to the samples for 100 seconds at a distance of 1 cm. The device's dosimetry was checked for accuracy (13).
The process of dispensing samples into the microplates was conducted within a laminar flow hood to ensure a sterile and dark environment. Additionally, the entire microplate was covered with thick black paper, with only the designated well openings punched out to a diameter of 0.8 cm. To prevent cross-contamination, samples were not placed in consecutive wells but in alternating wells. The minimum inhibitory concentration (MIC) was then determined for the photosensitizing substances (19).
The energy density of each irradiation can be calculated using the formula:
In this case, the energy density is equal to:
3.7. Staphylococcus Aureus Colony Counting
After the intervention, all samples were cultured on blood agar medium and incubated at 37°C for 24 hours (19). Following incubation, the number of bacterial colonies per milliliter (CFU/mL) was counted to assess bacterial growth.
3.8. Statistical Analysis
To compare the number of colonies among the study groups, the two-way random block design test was employed after confirming the normality of the data distribution.
4. Results
Table 1 presents the number of S. aureus colonies in different subgroups.
| Groups | Concentration | Numbers of Colonies |
|---|---|---|
| Staphylococcus aureus+ culture medium | NA | 1.5 × 10⁸ |
| Staphylococcus aureus+ MB (without irradiation) | 0.005 | 12 × 10⁴ |
| 0.002 | 84 × 10⁴ | |
| 0.001 | 44 × 10⁵ | |
| 0.0005 | 76 × 10⁵ | |
| 0.0002 | 59 × 10⁵ | |
| 0.0001 | UC | |
| 0.00005 | UC | |
| 0.00002 | UC | |
| 0.00001 | UC | |
| Staphylococcus aureus+ phycocyanin (without irradiation) | 0.02 | 10.8 × 104 |
| 0.01 | 42 × 10⁴ | |
| 0.005 | 26 × 10⁵ | |
| 0.002 | UC | |
| 0.001 | UC | |
| 0.0005 | UC | |
| 0.0002 | UC | |
| 0.0001 | UC | |
| 0.00005 | UC | |
| Staphylococcus aureus+ 660 nm laser | NA | 0.85 × 10⁸ |
| Staphylococcus aureus+ 660 nm laser + phycocyanin | 0.02 | 0.25 × 104 |
| 0.01 | 6.7 × 104 | |
| 0.005 | 88 × 10⁴ | |
| 0.002 | UC | |
| 0.001 | UC | |
| 0.0005 | UC | |
| 0.0002 | UC | |
| 0.0001 | UC | |
| 0.00005 | UC | |
| Staphylococcus aureus+ 660 nm laser + MB | 0.005 | 0 |
| 0.002 | 0 | |
| 0.001 | 68 × 10⁴ | |
| 0.0005 | 168 × 10⁴ | |
| 0.0002 | 146 × 10⁵ | |
| 0.0001 | 12 × 10⁶ | |
| 0.00005 | UC | |
| 0.00002 | UC | |
| 0.00001 | UC | |
| Staphylococcus aureus+ AMP (without irradiation) | NA | 0.15 × 10⁸ |
Abbreviations: MB, methylene blue; AMP, ampicillin.
The results demonstrated that laser irradiation combined with MB at concentrations of 0.005 and 0.002 mg/mL significantly reduced the number of S. aureus colonies more effectively than other subgroups (mean reduction = 100%, CI: [95%, 100%]; P-value < 0.01). This combination completely eradicated all S. aureus microorganisms, reducing the colony count to zero. Additionally, MB alone, without laser irradiation, was able to destroy all colonies at concentrations of ≥ 0.01 mg/mL, significantly reducing the colony count compared to other subgroups (P-value < 0.05), with a mean reduction of approximately 90% (CI: [80%, 100%]).
Furthermore, the study showed that laser irradiation combined with MB at concentrations of 0.001, 0.0005, and 0.0002 mg/mL, as well as with phycocyanin at concentrations of 0.02, 0.01, and 0.005 mg/ml, reduced the number of colonies. Phycocyanin alone decreased colony-forming units (CFUs) by approximately 30% (CI: [10%, 50%]) at higher concentrations, but its effect was less pronounced compared to MB. However, laser irradiation alone, laser irradiation combined with phycocyanin at concentrations ≤ 0.002 mg/mL, and laser irradiation combined with MB at concentrations ≤ 0.0001 mg/mL did not significantly reduce the number of colonies (P-value > 0.05).
5. Discussion
For the first time, this study compared the impact of phycocyanin and MB on the antibacterial effects of PDT. The results indicate that MB exhibits significantly greater antibacterial effects against S. aureus compared to phycocyanin, both with and without PDT. In a study by Virych et al. (20), a 60% reduction in S. aureus colonies was observed following laser irradiation with MB, with the 660 nm wavelength showing the highest efficacy among the lasers tested. Similarly, Tanev demonstrated that MB generates more ROS when exposed to laser light, further emphasizing the importance of laser wavelength. Additional research by Rineh et al. (21) found that a 668 nm laser could effectively kill S. aureus when used with MB. These findings support the conclusion that MB is an effective photosensitizer both alone and when combined with other agents, such as sodium citrate, silver, or zinc oxide nanoparticles, which have been shown to enhance MB’s antibacterial properties. In the present study, MB completely eradicated S. aureus colonies when irradiated with a 660 nm laser and significantly reduced colony numbers even without laser treatment, underscoring its strong antimicrobial potential (22-24).
Conversely, phycocyanin demonstrated much weaker antibacterial effects in this study. Although phycocyanin has been widely utilized in cancer treatment and has shown antimicrobial potential, few studies have assessed its effectiveness against S. aureus. One study by Chakroun et al. (25) reported that phycocyanin alone possesses antibacterial effects on S. aureus, but results have been inconsistent across studies. Several factors may explain these discrepancies. A key factor could be phycocyanin's optimal light absorption range, which lies between 580 - 630 nm. Since this study employed a 660 nm laser, phycocyanin may not have absorbed sufficient light energy to generate the ROS needed for bacterial eradication. Additionally, S. aureus produces acidic byproducts under certain growth conditions, and phycocyanin is sensitive to low-pH environments, where it tends to aggregate and lose its efficacy. These environmental factors, combined with variations in experimental design, such as differences in bacterial growth conditions, light exposure durations, and photosensitizer concentrations, likely contribute to the varying outcomes observed in studies investigating phycocyanin’s antibacterial effects (24-26).
It is also essential to compare the mechanisms of ROS production between MB and phycocyanin, as this could explain their differing antibacterial efficacies. When activated by laser light, MB generates a substantial amount of ROS, including singlet oxygen, which is highly toxic to bacterial cells. This ROS generation is well-documented and constitutes the primary reason for MB’s effectiveness in PDT. In contrast, while phycocyanin has been shown to produce ROS, the efficiency and types of ROS generated may differ. Phycocyanin primarily absorbs light in the 580-630 nm range, and its ability to generate ROS under the 660 nm laser used in this study may have been suboptimal. This disparity in ROS generation mechanisms and efficiencies likely accounts for MB’s superior antibacterial performance compared to phycocyanin (11, 27, 28).
The combination of low-power lasers and photosensitizers in PDT represents a promising approach for eradicating bacteria and promoting wound healing. One of PDT’s key advantages over conventional antibiotics is its ability to circumvent the development of bacterial resistance. Unlike antibiotics, which target specific bacterial pathways, PDT operates by generating ROS that cause widespread oxidative damage to bacterial cells, affecting proteins, lipids, and DNA. This non-specific mechanism significantly reduces the likelihood of bacteria, including S.aureus, developing resistance to PDT. Achieving resistance to ROS would require multiple simultaneous genetic changes, a challenge that is exceedingly difficult for bacteria to overcome (29-31).
Despite the promising results for MB, there are still no studies examining the simultaneous effects of PDT and phycocyanin on S. aureus, and the limited research on phycocyanin's antimicrobial properties presents conflicting data. For instance, Safari et al. found that phycocyanin is more effective against gram-positive bacteria, such as Streptococcusiniae, but less effective against gram-negative species (32). These discrepancies may arise from variations in experimental conditions or differences in bacterial physiology, highlighting the need for further investigation.
While this study provides valuable insights, it has limitations. The experiments were conducted in vitro under controlled conditions, and the results may not fully translate to clinical settings. Consequently, animal and clinical trials are recommended to evaluate the efficacy of PDT with MB or phycocyanin in real-world scenarios. To effectively implement this method in clinical practice, optimizing the dosage of photosensitizers and calibrating light exposure will be crucial. Photodynamic therapy holds particular promise for treating localized infections, such as chronic wounds, infections surrounding medical implants, and nasal decolonization in high-risk patients. Monitoring bacterial load and tissue response during treatment will be essential to ensure therapeutic success and prevent damage to healthy tissues (33, 34).
5.1. Conclusions
In the present study, MB was able to destroy all S. aureus colonies at concentrations of 0.01 mg/mL or higher without laser irradiation. Additionally, 0.002 and 0.005 mg/mL concentrations of this photosensitizer completely eradicated all colonies when combined with laser irradiation. However, phycocyanin only insignificantly reduced the number of colonies. Therefore, MB appears to be a more effective photosensitizer for PDT compared to phycocyanin. Unfortunately, no prior studies have compared the antibacterial effects of phycocyanin and MB in PDT, making this study the first of its kind. Thus, further research on this topic is recommended.