1. Background
Multiple sclerosis (MS) is a chronic, inflammatory, and autoimmune disorder that primarily affects the central nervous system (CNS), leading to demyelination, axonal loss, and neurodegeneration (1). The disease presents with a wide spectrum of neurological symptoms, including motor, sensory, and cognitive impairments, depending on the location of CNS lesions. Multiple sclerosis is one of the most common causes of neurological disability in young adults, affecting more than 2.8 million people worldwide, with a higher prevalence in women (2). The unpredictable progression of the disease, coupled with its broad range of physical and psychological complications, often results in significant disability and a diminished quality of life (3).
Among the various manifestations of MS, spasticity is one of the most common and disabling symptoms, affecting approximately 80% of patients at some point during their disease course (4). Spasticity is characterized by increased muscle tone and involuntary muscle contractions that interfere with voluntary movement. It results from abnormal motor signals from the CNS, often triggered by rapid passive joint movements (5). This condition can cause discomfort, pain, limited mobility, muscle stiffness, and contractures, significantly impairing daily activities such as walking and self-care and contributing to a decline in overall quality of life (6). Managing spasticity is a critical component of MS care, as failure to control it can lead to long-term physical disability and exacerbate other MS-related symptoms.
One therapeutic approach that has gained attention for managing spasticity in MS is the use of botulinum toxin (BT). Botulinum toxin type A (BT-A), a neurotoxin produced by Clostridium botulinum, works by inhibiting the release of acetylcholine at the neuromuscular junction, resulting in temporary and reversible muscle paralysis (7). The BT-A has been shown to effectively reduce muscle hyperactivity in various neurological conditions, including stroke, cerebral palsy, and MS (8). The intramuscular administration of BT-A provides a targeted approach to alleviating spasticity by relaxing overactive muscles, thereby improving functional outcomes and reducing discomfort (9).
Although the efficacy of BT-A in treating spasticity has been demonstrated in several studies, its therapeutic effects are transient, typically lasting between 3 and 6 months (10). Consequently, the long-term effectiveness of BT-A in managing MS-related spasticity remains a topic of debate. While some studies report significant improvements in spasticity and related functional outcomes, others have observed only modest benefits, raising questions about the consistency of its efficacy (11). Additionally, variability in treatment response and the diverse methodologies employed across studies contribute to inconsistent findings (12). This variability underscores the need for a comprehensive evaluation of the available evidence to inform clinical guidelines and optimize treatment strategies.
A systematic review and meta-analysis provide a critical synthesis of existing data, offering a more definitive assessment of BT’s efficacy and safety in reducing spasticity in MS patients. Several meta-analyses have previously evaluated the effect of BT on spasticity in MS patients. However, their findings have been inconsistent, likely due to variations in treatment protocols, dosages, patient populations, and follow-up durations. Additionally, many of these studies included a limited number of randomized controlled trials (RCTs), which has contributed to the lack of definitive conclusions.
Given the heterogeneity of findings in the literature and the absence of consensus on the optimal use of BT-A for spasticity in MS, we conducted this systematic review and meta-analysis to update and refine the pooled efficacy of BT in reducing spasticity and improving functional outcomes in this patient population. Our study employs the PICO framework to define the research focus:
- P (Population): Patients with MS experiencing spasticity
- I (Intervention): Botulinum toxin injections
- C (Comparison): Various standard treatments or control groups
- O (Outcomes): (1) Main outcomes: Changes in spasticity measured by the Modified Ashworth Scale (MAS) and overall quality of life; (2) secondary outcomes: Changes in the Expanded Disability Status Scale (EDSS) and any reported side effects.
2. Objectives
Understanding the efficacy of BT in this context is essential for optimizing treatment strategies and improving patient outcomes. This review seeks to provide a comprehensive assessment of the available literature on the subject and identify gaps for future research. By synthesizing data from multiple studies, we aim to elucidate the effectiveness of this treatment approach and highlight areas for further investigation.
3. Methods
3.1. Search Strategy
Two independent researchers systematically searched the PubMed, Embase, Web of Science, and Google Scholar databases up to April 2023 to identify relevant articles. The search strategy employed a combination of MeSH terms and keywords such as "Multiple Sclerosis", "Spasticity", "Botulinum Toxin", "BT-A", and "Treatment Outcome". Boolean operators (AND/OR) were used to refine the search results.
References were managed and organized using EndNote X9, while Rayyan QCRI software facilitated the screening process for study selection. The search strategy adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, and a PRISMA checklist was utilized to ensure transparency and completeness in reporting each stage of the review (13).
The keywords, including MeSH terms and their synonyms, used across different databases included:
- ("Multiple Sclerosis" [MeSH] OR "Disseminated Sclerosis") AND
- ("Botulinum Toxins" [MeSH] OR "Clostridium botulinum Neurotoxins")
Additionally, the reference lists of initially selected articles were reviewed to capture any relevant studies that may have been missed.
3.2. Study Selection Criteria
Randomized controlled trials and observational studies (e.g., cohort studies) reporting on the efficacy of BT in treating spasticity in MS patients were included. Grey literature, such as conference papers, unpublished studies, and dissertations, was also reviewed to ensure a comprehensive evaluation. Grey literature was identified through searches in Google Scholar and by examining the references of relevant articles. This approach helped to minimize publication bias and provided a broader representation of evidence.
Studies were excluded if they combined BT with other medications or did not provide a quantitative measure of BT’s efficacy. Additionally, case reports, letters, animal studies, and articles in languages other than English were excluded due to practical limitations in translating and verifying non-English texts. Only English-language articles meeting the eligibility criteria were included in the final selection. Grey literature was considered if it adhered to the inclusion criteria and offered sufficient data for analysis.
3.3. Data Extraction and Quality Assessment
Data extracted from each study included the total number of participants, the first author’s name, year of publication, country of origin, mean age, disease duration, EDSS scores, and the main findings. The primary outcome was spasticity reduction, predominantly assessed using the MAS and changes in overall quality of life. Secondary outcomes included EDSS scores and any adverse effects reported following the intervention.
Two independent reviewers (OM and SV) conducted the data extraction, and discrepancies were resolved through discussion with a third researcher (AT). The risk of bias was assessed using the Cochrane Collaboration’s tool for RCTs and the Newcastle-Ottawa Scale (NOS) for cohort studies (14, 15).
3.4. Statistical Analysis
Standardized mean differences (SMDs) for the MAS and their corresponding standard deviations (SDs) were used to calculate effect sizes in the meta-analysis. When data were reported as medians (range), they were converted to means (SD) using the following formulas:
To address potential heterogeneity across studies, both fixed-effects and random-effects models were employed based on the degree of heterogeneity observed. A fixed-effects model was applied when heterogeneity was low, whereas a random-effects model was used in cases of significant heterogeneity. Heterogeneity was assessed using Cochran’s Q-test and quantified with the I² statistic, which was interpreted as follows: (1) 0 - 25%: Low heterogeneity; (2) 26 - 50%: Moderate heterogeneity; (3) 51 - 75%: Substantial heterogeneity; (4) 76 - 100%: Considerable heterogeneity.
Sensitivity analyses were conducted to evaluate the robustness of the findings by excluding studies with a high risk of bias or those employing differing methodologies. Publication bias was assessed using funnel plots and Egger’s test. All statistical analyses were performed using STATA software, version 14.0 (Stata Corporation, College Station, TX, USA), with P-values less than 0.05 considered statistically significant.
4. Results
4.1. Study Selection
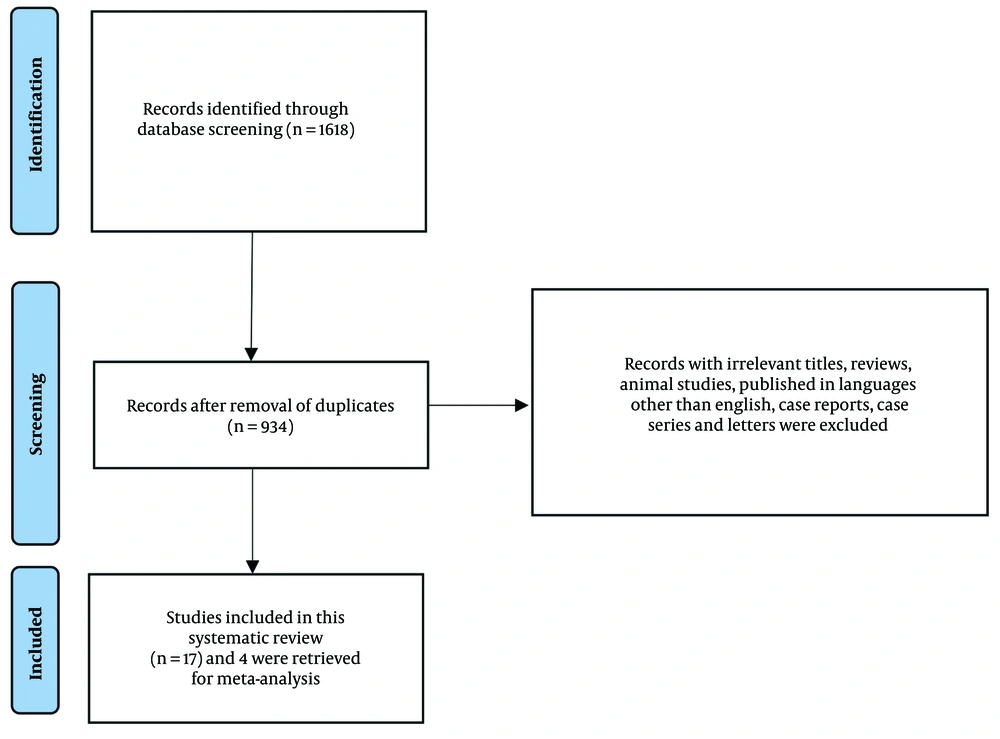
The initial search identified 1,618 articles, of which 684 were duplicates and subsequently removed. The remaining 934 articles were screened based on their titles and abstracts, with full-text reviews conducted when necessary. Ultimately, 17 articles met the inclusion criteria and were included in the systematic review, while data from 4 studies were eligible for inclusion in the meta-analysis (3, 7, 8, 16). The study selection process is illustrated in the PRISMA flowchart (Figure 1).
4.2. Study Characteristics
The demographic details of the included studies are summarized in Table 1. A total of 958 patients were enrolled across these studies. The mean age of participants ranged from 38 to 53 years, with follow-up durations varying between 1 and 36 months. Among the 17 studies, 9 were conference papers, 4 were RCTs, and 4 were cohort studies. These studies were published between 2000 and 2021 and were conducted in Germany, Poland, the Czech Republic, France, Italy, the USA, and the UK (Table 2).
| Study | Country | Year | Study Design | Dosing | Anatomic Location | MS Category | F/M Ratio | Age | Disease Duration | EDSS | Follow-Up Duration (Mon) | Main Findings | Side Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sartori et al. (17) | Italy | 2021 | Cohort study | Onabotulinum toxin A/(50 - 300 U), Incobotulinum toxin A/(20 - 300 U) | Upper limbs | 28/RRMS: 8, PPMS: 5, SPMS: 15 | 13/15 | 52.8 (10.3) | 15.62 (4.6 - 39.1) | 7 (2 - 8) | 1 | BT is effective in treating spasticity (i.e. reducing MAS) and has a satisfactory safety profile. MAS median (range); baseline: 3 (1 - 4); week 4: 2 (1 - 4) | One patient reported side effect |
| 2. Marinaro et al. (3) | Italy | 2021 | RCT | BT A/(50 – 300 U) | Lower limb (triceps surae, gastrocnemius, soleus) | 16/NR | 6/10 | 45 (8.17) | NR | 5.93 (0.75) | 3 | The study highlights the efficacy of BT treatment of focal spasticity in MS patients. MAS score mean (SD): Baseline: 2.56 (0.81); week 4: 1.96 (0.95); week 12: 1.90 (0.84) | None |
| 3. Moccia et al. (18) | Italy | 2020 | Cohort study | Abobotulinum toxin A/(30 – 1500 U), onabotulinum toxin A/(10 – 270 U), inco botulinum toxin A/(10 – 400 U) | Upper limb (adducted shoulder, extended elbow, flexed elbow, flexed wrist, clenched fist, thumb-in-palm), lower limb (flexed hip, adducted thigh, extended knee, flexed knee, equine foot, flexed toes, hitch-hiker toe) | 386/RRMS:213, PPMS:88, SPMS:85 | 228/158 | 53.6 (10.9) | 18.7 (9.2) | 6.5 (2 - 9) | NR | BT is a satisfactory treatment for the management of a variety of spasticity-related symptoms in patients with MS. | Temporary asthenia/weakness: 2, hypophonia:One |
| 4. Francisco et al. (19) | USA | 2020 | Cohort study | Onabotulinum toxin A/(10 – 875 U) | Upper limb (clenched fist, flexted elbow, flexted wrist), lower limb (equinovarus foot, flexted knee, adducted thigh) | 119/NR | 83/36 | 53.1 (10.3) | NR | NR | 12 | Significant improvement was reported for spasticity regardless of etiology after BT injection. | Muscle weakness: 3, dry mouth:one, influenza-like illness:One |
| 5. Butera et al. (20) | Italy | 2018 | Conference paper | BT A/NR | NR | 15/Progressive MS:15 | NR | NR | NR | 6.5 (4 - 8) | 1 | Gait improvement was observed in 8 patients and 3 patients experienced postural changes. | NR |
| 6. Hlustik et al. (21) | Czech | 2017 | Conference paper | BT A/NR | Lower limb (bilateral leg) | 11/NR | 8/3 | 47.1 (9.1) | NR | 6.5 | 3 | BT injection decreased spasticity. | NR |
| 7. Leblong et al. (22) | France | 2017 | Conference paper | Incobotulinum toxin A/200U | Lower limb (triceps surae) | 22/NR | NR | 48.2 (12 | NR | 4.2 | 3 | BT is useful for treating the focal spasticity of the triceps surae and it results in gait improvement reduces fatigability and enhances endurance. | NR |
| 8. Coghe et al. (23) | Italy | 2016 | Conference paper | BT/NR | Lower limb (tibialis posterior, soleus, gastrocnemius lateralis and medialis) | 14/NR | 10/4 | 50.4 (12.3) | NR | 4.9 (1.3) | 1 | The NRS was reduced by 1.14 after the BT injection. of 14 patients, 8 reported improvements in their symptoms. | NR |
| 9. Gallien et al. (24) | France | 2016 | Conference paper | Incobotulinum toxin A/200U | Upper limb (triceps surae) | 28/NR | NR | 48.2 (12) | NR | 4.2 (4.7 med) | 3 | The results were in favor of BT injection for focal spasticity of the triceps surae and showed a significant improvement in gait and posture. | NR |
| 10. Schramm et al. (25) | Germany | 2014 | Cohort study | Onabotulinum toxin A/(2 -780U) | Upper limb, lower limb | 52/NR | NR | 49.83 (10.78) | 12.52 (8.90) | NR | NR | The data demonstrated a high efficacy and safety profile BT injection for spasticity. MAS for upper limb [mean (SD)]: Baseline: 2.22 (0.79); effect: 0.07 (0.44); MAS for lower limb: Baseline: 2.59 (0.78); effect: 0.09 (0.73) | Transient weakness of injected muscles |
| 11. Paoloni et al. (7) | Italy | 2013 | RCT | BT A/(100 – 300 U) | Lower limb (rectus femoris, gastrocnemius medial and lateral, soleus) | 14/SPMS:14 | 10/4 | 50.6 (8.9) | NR | 5.5 (4.6) | 5.5 | BT injection reduces spasticity in MS patients and resolves fatigue. MAS for knee: Median (range): Baseline: 4.0 (3.0 - 4.0); week 10: 3.0 (2.0 - 3.0); week 22: 3.0 (2.0 - 4.0); MAS for ankle: Median (range): Baseline: 4.0 (4.0 - 4.0); week 10: 3.0 (3.0 - 4.0); week 22: 4.0 (3.0 - 4.0) | None |
| 12. Ochudło (26) | Poland | 2012 | Conference paper | BT A/200U | Lower limb (hip adductor) | 22/PPMS:22 | NR | NR | 8 | 7.3 | 36 | The majority of patients (27) reported improvement in spasticity. BT reduced the spasticity in hip adductors and relieved the pain associated with PPMS. | Muscle weakness: 2 |
| 13. Gallien et al. (28) | France | 2012 | Conference paper | BT A/NR | Upper limb (triceps surae), lower limb (adductors, hamstrings) | 126/NR | 85/41 | 49.4 (11) | NR | 5.8 (1.7) | NR | The patient experienced satisfactory outcomes for the most part. | NR |
| 14. Giovannelli et al. (27) | Italy | 2007 | RCT | BT A/(100 - 300 U) | Upper limb (flexor digitorum superficialis, flexor carpi radialis, flexor carpi ulnaris), lower limb (tibialis posterior, gastrocnemius medial and lateral, soleus) | 18/SPMS:18 | 16/2 | 48.1 (7.5) | NR | 6.0 (1.1) | 3 | A significant improvement in spasticity was also observed via the visual analog scale. MAS: Mean (SD): Baseline: 3.61 (0.50); week 2: 3.22 (0.55); week 4: 3.33 (0.60); week 12: 3.33 (0.60) | NR |
| 15. Pappert (29) | USA | 2007 | Conference paper | BT B/(25000 – 45000 U) | Lower limb (bilateral lower-limb adductor) | 24/NR | 14/10 | NR | NR | NR | 4 | Safety data suggests a starting dose of 30000 U for lower-limb adductor spasticity. | Dry mouth:11, dysphagia:7, constipation:4 |
| 16. Restivo et al. (30) | Italy | 2003 | Conference paper | BT A/(50 – 120 U) | Upper limb (forearm finger flexor, flexor ulnaris carpi), lower limb (gastrocnemius, small flexor, foot) | 5/RRMS:3, SPMS:2 | 2/3 | 38.8 (25 - 52) | 9.2 (3 - 16) | 5.4 (4 - 6.5) | 4 | Mean values of pain intensity score and the daily number of painful tonic spasms were significantly improved after BT injection except for one patient. | None |
| 17. Hyman et al. (31) | UK | 2000 | RCT | Abobotulinum toxin A/(500 U); Abobotulinum toxin A/(1000 U); Abobotulinum toxin A/(1500 U) | Lower limb (adductor magnus, adductor longus, adductor brevis) | 21/NR; 20/NR; 17/NR | 16/5; 9/11; 9/8 | 47.0 (12.2); 54.0 (9.9); 46.8 (10.3) | 16.5 (7.3); 22.9 (10.6); 21.2 (10.6) | 8.00 (median); 7.50 (median); 7.50 (median) | 1 | Reduced spasm frequency and improved muscle tone were observed after BT injection. The proportion of pain-free patients increased at week 4, and the administration of BT reduced the degree of hip adductor spasticity associated with MS. MAS median (500 U): Baseline: 8.5; week 4: 4.0; MAS median (1000 U): Baseline: 16.0 ; week 4: 12.0; MAS median (1500 U): Baseline: 14.0; week 4: 8.0 | Hypertonia:22, muscle weakness: 14, fatigue: 7, urinary tract infection: 5, headache: 5, micturition frequency: 5, back pain:5, diarrhoea:5, arthralgia:3, gait abnormal:3, abscess: 3, constipation:3, infection: 3, influenza-like symptoms: 3, nausea: 3, skin disorder: 3, abdominal pain: 2, fever: 2, URTI: 2V |
Abbreviations: F/M ratio, female-to-male ratio; EDSS, Expanded Disability Status Scale; MAS, Modified Ashworth Scale; NRS, Numeric Rating Scale; NR, not reported; RRMS, PPMS and SPMS, relapsing-remitting, primary-progressive, and secondary-progressive MS variants; BT, botulinum toxin; RCT, randomized controlled trial.
The therapeutic variants of BT used included onabotulinum, incobotulinum, and abobotulinum toxins. The injection site was the lower limb in 7 studies and the upper limb in 4 studies. A wide range of side effects was reported by the patients, including headache, dry mouth, dysphagia, and constipation, with muscle weakness being the most commonly reported complication following BT injection.
| Authors | Year | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Potential Threats to Validity |
|---|---|---|---|---|---|---|---|
| Marinaro et al. (3) | 2021 | HRB | HRB | HRB | URB | LRB | HRB |
| Paoloni et al. (7) | 2013 | LRB | HRB | LRB | URL | LRB | URB |
| Giovannelli et al. (27) | 2007 | LRB | HRB | URL | LRB | URL | HRB |
| Hyman et al. (31) | 2000 | LRB | LRB | LRB | LRB | HRB | URB |
Abbreviations: HRB, high risk of bias; LRB, low risk of bias; URB, unclear risk of bias.
The quality assessment (QA) of observational studies is shown in Table 3.
The QA scores range from 0 to 10, where: (1) 0 - 3 indicates low quality; (2) 4 - 6 indicates moderate quality; (3) 7 - 10 indicates high quality.
This scoring system reflects the methodological rigor and reliability of each study, with higher scores indicating a lower risk of bias and stronger study quality.
4.3. Qualitative Synthesis of Non-meta-analyzed Studies
In addition to the 4 studies included in the meta-analysis, 13 studies were identified that provided valuable insights but could not be quantitatively analyzed. A qualitative synthesis of these studies is presented below.
4.3.1. Study Findings
The majority of the non-meta-analyzed studies reported on the effectiveness of BT in reducing spasticity levels among MS patients, with varied methodologies and outcome measures. Some studies focused on specific demographics, such as age or severity of MS, while others explored different administration techniques and dosages.
4.3.2. Reported Outcomes
Many of these studies indicated significant improvements in spasticity, as assessed by various scales, including the MAS and the Tardieu Scale. Additionally, several studies highlighted improvements in overall quality of life and functional mobility post-treatment.
4.3.3. Adverse Effects
The non-meta-analyzed studies frequently reported side effects similar to those observed in the meta-analyzed studies, with muscle weakness being a common concern. However, some studies noted unique adverse effects, such as transient dysarthria and localized pain at the injection site.
4.3.4. Limitations and Gaps
While many studies reported positive outcomes, several highlighted limitations such as small sample sizes, short follow-up durations, and the absence of control groups. These factors limit the generalizability of their findings and underscore the need for more rigorous RCTs.
4.3.5. Quality Assessment of Grey Literature
It is important to note that the grey literature, which includes conference papers and non-peer-reviewed studies, was not subjected to the same rigorous QA as the peer-reviewed studies. The quality of these studies varied significantly, and their inclusion without proper evaluation may introduce potential biases in the overall findings of the review. Future research should prioritize the systematic evaluation of grey literature to assess its reliability and relevance, as it may influence the implications of treatment effectiveness and recommendations.
4.3.6. Conclusion from Qualitative Synthesis
The qualitative analysis of these 13 studies reinforces the notion that BT can be an effective treatment for spasticity in MS patients. However, it also emphasizes the necessity for further research to establish standardized protocols and investigate the long-term effects and optimal treatment regimens.
4.4. Efficacy of Botulinum Toxin for Treating Spasticity in Multiple Sclerosis
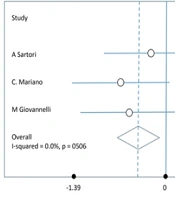
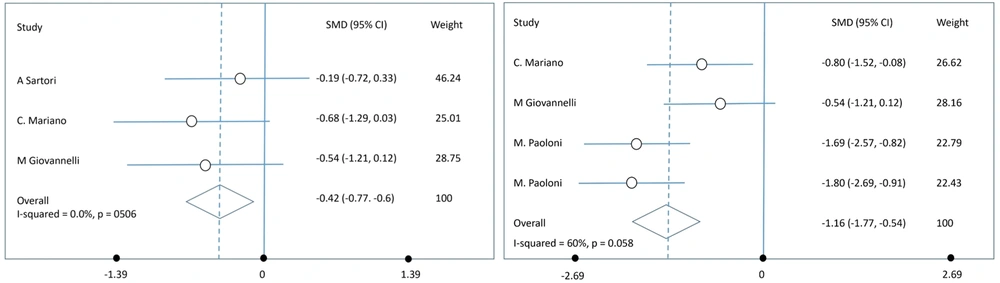
The meta-analysis performed on 4 studies using a random-effects model revealed a significant reduction in spasticity, as measured by the MAS, following BT injections. The pooled effect size at week 4 was a SMD of -0.42 (95% CI: -0.77 to -0.6), indicating a moderate reduction in spasticity. At week 12, the SMD increased to -1.16 (95% CI: -1.77 to -0.54), suggesting a more substantial reduction in spasticity over time (Figure 2).
A sensitivity analysis was conducted to assess the robustness of the findings. This analysis involved excluding studies that were deemed to have a high risk of bias or those with significant methodological differences. The results of the sensitivity analysis confirmed the robustness of the initial findings, showing that the overall effect size was not significantly altered. At week 4, there was no significant heterogeneity among the studies (I² = 0%), suggesting consistent findings. However, by week 12, moderate heterogeneity was observed (I² = 60%), indicating some variability in the results, likely due to differences in sample sizes, follow-up durations, or BT variants used.
5. Discussion
5.1. Summary of Key Findings
In this systematic review and meta-analysis, we aimed to evaluate the efficacy of BT for treating spasticity, using the MAS, and improving disability status according to the EDSS in patients with MS. Our rigorous study selection process identified 17 articles that met the inclusion criteria for the systematic review, with data from 4 of these studies included in the meta-analysis.
The primary outcome measure for this meta-analysis was the MAS, a widely used tool for assessing spasticity. Our findings consistently demonstrate a significant reduction in spasticity scores following BT injection, indicating the potential of this treatment to provide symptomatic relief. The evidence underscores the effectiveness of BT in decreasing spasticity (via MAS) and improving disability outcomes (via EDSS). Notably, the efficacy was observed to be greater at three months post-injection (SMD = -1.16) compared to four weeks (SMD = -0.42), suggesting that the beneficial effects of BT may improve over time. Therefore, BT injections can be a valuable therapeutic option in the clinical management of MS-related spasticity. It is advisable for clinicians to tailor the BT dosage based on individual MAS and EDSS scores to achieve optimal therapeutic effects.
5.2. Comparison with Previous Studies
These results align with the findings of Marinaro et al., who reported significant reductions in MAS scores at both four and twelve weeks following BT injection in MS patients, with more pronounced effects at the later time point (3). The pathophysiology of spasticity in MS involves demyelination within the CNS, particularly affecting descending spinal pathways, such as the corticospinal tract, which plays a crucial role in motor control. In our review, the studies consistently demonstrated that BT administration led to marked improvements in spasticity, with a substantial number of participants reporting reductions in MAS scores. For instance, in the study by Snow et al. (32), participants exhibited a remarkable 78% improvement in spasticity following BT injections in the adductor muscles. Similarly, later studies reinforced these findings, indicating that patients receiving BT experienced not only reduced muscle tone but also improved functional mobility, which is critical for enhancing overall quality of life (4).
5.3. Clinical Implications
Several studies included in this systematic review reported a significant reduction in MAS scores following BT injections, indicating a decrease in spasticity severity. Sartori et al. observed a decrease in MAS from 3 to 2 at week 4 post-injection, demonstrating a marked reduction in spasticity (17). In Marinaro et al., the MAS score significantly dropped from an initial mean of 2.56 to 1.90 at 12 weeks, showing sustained improvement in muscle tone (3).
Improvements in EDSS scores, reflecting enhanced physical functioning and reduced disability, were reported across various included studies. Moccia et al. documented significant improvements in EDSS scores after BT treatment, suggesting reduced disability severity and enhanced overall motor performance (18). Similarly, Hyman et al. found a positive impact of BT on lowering EDSS scores, affirming its role in improving functional outcomes in MS patients with spasticity (31).
Given the impact of spasticity on quality of life, the significant reduction observed in our analysis suggests that BT can be an effective component of a multidisciplinary approach for managing spasticity in MS patients.
Despite the promising results, there remains no consensus on the optimal dosing of BT for spasticity in MS patients. Phadke et al. (33) suggest that MS patients may require higher doses of BT compared to those with stroke or cerebral palsy, while Schramm et al. (25) found no significant differences in effective dosing across various neurological disorders. Although serious adverse effects are rare, there is ongoing concern regarding the safety of BT injections. Careful consideration must be given before initiating therapy, as transient muscle weakness is the most commonly reported adverse effect, occurring in up to 35% of patients, particularly following high doses (e.g., 800 IU to 1000 IU).
5.4. Limitations of the Study
This study, however, is subject to several limitations. Firstly, the relatively small number of RCTs included in the meta-analysis limits the robustness of our findings and their generalizability. The inclusion of grey literature, such as conference papers, raises concerns about the quality of evidence and the potential for bias. Grey literature is often associated with lower methodological rigor compared to published studies, which could affect the strength of our conclusions.
Additionally, the heterogeneity observed at week 12 indicates the presence of potential confounding factors that may have influenced treatment outcomes. Variability in study designs, participant characteristics, and treatment protocols (such as dosage and injection sites) makes it difficult to draw definitive conclusions about the efficacy of BT injections in spasticity management. Variations in BT dosing (e.g., ranging from 50 to 300 U) and anatomical target areas further complicate the interpretation of results.
Another limitation is the short follow-up durations in many of the included studies. While many studies had follow-up periods ranging from a few weeks to several months, this may not be sufficient to assess the long-term effects and sustainability of treatment outcomes. The impact of BT injections on patients' functional mobility and quality of life, particularly in terms of enduring effects and potential side effects over time, remains unclear.
Furthermore, the inclusion of studies with a wide variety of MS subtypes (including relapsing-remitting MS, progressive forms, and different levels of spasticity severity) introduces additional complexity in interpreting results across the full patient spectrum. It is possible that treatment outcomes may differ based on these clinical factors, but this was not always accounted for in the analysis.
Lastly, the potential for publication bias must be acknowledged, as studies with positive results are more likely to be published, skewing the overall findings toward more favorable outcomes. Additionally, we may have inadvertently missed relevant studies due to publication language restrictions or the exclusion of unpublished data. Another potential issue is the possibility of data extraction bias. Although efforts were made to ensure consistency and accuracy in data extraction, inconsistencies or errors in reporting across the included studies could have affected the overall analysis. Furthermore, the heterogeneity in study designs and patient populations meant that some important subgroup analyses, such as the impact of BT on different MS subtypes, were not feasible.
5.5. Recommendations for Future Research
Future research should aim to address these limitations by conducting larger, multicenter RCTs with standardized protocols for BT dosing and administration, as well as longer follow-up durations. These studies should consider diverse patient populations to enhance the generalizability of findings. Additionally, investigating the long-term efficacy and safety of BT treatment is crucial, as this will help establish the durability of its effects and identify potential complications over extended periods. Furthermore, exploring the role of adjuvant therapies, such as physiotherapy or occupational therapy, may provide valuable insights into optimizing treatment outcomes for MS patients with spasticity. Understanding the mechanisms underlying the development of tolerance to BT could also guide the development of strategies to maintain its effectiveness over time, ensuring sustained benefits for patients.
5.6. Conclusions
In summary, the current study provides strong evidence supporting the efficacy of BT in reducing spasticity among patients with MS, with significant reductions in spasticity observed at both 4 and 12 weeks post-injection. The findings suggest that BT injections can serve as a valuable therapeutic option for managing spasticity-related symptoms, ultimately enhancing the quality of life for affected individuals. However, to solidify these claims and inform clinical decision-making regarding the use of BT in MS patients, further research is essential. Future studies should aim to include larger sample sizes, standardized protocols, and considerations of potential confounders to better assess the long-term efficacy and safety of BT treatment. By addressing these gaps, researchers can contribute to a more robust understanding of BT's role in the management of spasticity in MS, ultimately leading to improved patient outcomes.