1. Context
Diabetes mellitus poses a significant public health challenge, characterized by its high prevalence, morbidity, and mortality (1). The rising prevalence of diabetes has led to increased complications, including foot ulcers, affecting roughly 15% of diabetic patients, and subsequent amputations, observed in 15 to 20% of patients with such ulcers (2). Diabetic foot ulcers (DFUs) represent a prevalent complication of uncontrolled diabetes, often stemming from diabetic peripheral neuropathy, heightened pressure on the foot, vascular issues, and traumatic injuries (3). The DFUs are divided into three categories: Neuropathic (commonly occurring in weight-bearing areas of the foot, such as metatarsal heads and the heel), ischemic (often located in areas around the lateral fifth metatarsal head, medial first metatarsal head, and tips of toes), and neuroischemic (a combination of the first two) (4, 5). The DFUs lead to disability, distress, activity disruption, and significant healthcare costs (6).
While standard treatments for DFUs include various dressings, debridement, offloading, revascularization surgery, and compression therapy (7), many DFUs do not respond to these methods. This lack of response highlights the need for more effective therapeutic approaches to expedite wound healing and reduce the burden of DFUs. Consequently, many studies are exploring therapeutic approaches to expedite wound healing. Modalities like electrical stimulation (ES), ultrasound therapy, low-level laser therapy (LLLT) or photobiomodulation (PBM), and shockwave therapy are being investigated as adjunct treatments for improving chronic wound healing, including DFUs (8, 9). Electrical stimulation and LLLT are the most commonly employed methods, demonstrating significant potential in treating DFUs (10). These modalities offer promising alternatives to standard care, particularly for patients who do not respond to conventional treatments.
It is important to note that patient responses vary depending on ulcer severity, comorbidities, and treatment adherence. Additionally, adjunctive therapies like physical therapy are crucial in managing DFUs by addressing functional limitations, improving mobility, and enhancing patient compliance with offloading strategies. These therapies, when combined with standard care, can further optimize outcomes and reduce the risk of ulcer recurrence. The ES is a safe and economical physiotherapy method that has shown effectiveness in healing DFUs. However, its clinical implementation necessitates staff training for correct electrode positioning and may face obstacles in resource-limited environments due to equipment needs. Similarly, PBM, while non-invasive, demands precise dosing protocols and has higher initial costs for laser devices. Both treatments generally require several sessions, potentially posing challenges for patient adherence.
While the neurophysiological mechanisms behind ES's wound-healing effects remain unclear, research suggests ES enhances wound healing through antibacterial effects (11), increases blood circulation (12), and promotes cell migration by replicating the body's natural electric currents at the wound site (10, 13). A recent systematic review and meta-analysis by Zheng et al. (14) found that combining ES with standard care leads to accelerated healing and reduced ulcer size in DFUs compared to treatment with routine care alone.
The LLLT, recently known as PBM, is a non-invasive physiotherapy technique that utilizes low-power, monochromatic, and coherent light, typically ranging between 10 and 90 mW/cm2, with an intensity of 1 - 4 J/cm2 when applied to treatment areas (15). Various systematic reviews and network meta-analyses have investigated the effectiveness of LLLT in healing ulcers among individuals with DFUs (16-18). These studies have consistently highlighted the therapeutic advantages of LLLT in diabetic wound healing, including increased ulcer healing rates, reduced ulcer size, enhanced granulation tissue formation, and relief from DFU-related pain. The precise mechanisms driving the healing effects of LLLT for DFUs remain elusive. However, reports suggest that these effects may be attributed to increased collagen and extracellular matrix synthesis (19, 20), improved local blood circulation (21), heightened ATP production (20), increased growth factor secretion (22), reduced levels of matrix metalloproteinases (MMPs), and decreased activity of inflammatory cytokines (23).
Numerous clinical studies have demonstrated that ES and LLLT expedite the healing of DFUs and exhibit superiority over placebos and standard care. For over five decades, ES devices have been used to facilitate wound healing by utilizing Direct Current and mono and biphasic pulsed current waveforms. In recent years, newer technologies, including bioelectric dressing-like devices, have emerged as potential tools in this endeavor (24).
The LLLT was discovered by Mester et al. in 1968 (25-27). Mester's research showed that LLLT has promising effects on cell function and tissue repair, particularly in enhancing wound healing. Since then, advancements in laser technology, such as light-emitting diodes (LEDs), broadband light using filters, and high-power lasers, have further expanded the potential applications of LLLT in wound management.
2. Objectives
However, despite the extensive use of ES and LLLT in chronic wounds, their effectiveness in treating DFUs remains relatively unexplored. Notably, no clinical trials, published reviews, or meta-analyses have yet compared the effectiveness of ES and LLLT in DFU treatment. This gap in the literature underscores the need for a comprehensive evaluation of these therapies to guide clinical decision-making. Furthermore, the variability in patient responses to these therapies, influenced by factors such as ulcer severity, patient comorbidities, and adherence to treatment protocols, underscores the need for a comprehensive evaluation. This variability highlights the ongoing challenges in the field and emphasizes the importance of synthesizing existing evidence to guide clinical decision-making. Therefore, this systematic review aims to evaluate the relative efficacy of these two therapies for managing DFUs and provide an evidence-based foundation for clinical decision-making in wound management.
3. Data Sources
3.1. Study Design and Registration
This literature review, conducted until April 29, 2024, followed a structured approach involving a systematic review of scientific articles and a meta-analysis, adhering to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (28). This systematic review and meta-analysis was not registered in a public trial registry because it is a secondary analysis of existing studies, and registration is not typically required for systematic reviews and meta-analyses. The research process consisted of several distinct phases. Initially, we formulated the research topic and devised a comprehensive search strategy. Subsequently, we systematically screened and curated relevant articles from various databases. We rigorously assessed these articles based on predefined inclusion and exclusion criteria. Additionally, a qualitative evaluation was performed, and the review culminated in a comprehensive statistical analysis.
3.2. Search Strategy
We conducted an extensive search for relevant studies by systematically exploring various international databases, including Medline (through PubMed), Web of Science, Cochrane, Scopus, and Pedro. Our search was not limited by time, extending until April 29, 2024. Furthermore, an extensive examination of pertinent studies' reference lists was undertaken to identify all extant articles within this domain. The search strategy employed a combination of MeSH terms and keywords such as "DFUs," "Electrical Stimulation," "Photobiomodulation," "Low-Level Laser Therapy," "Low-Power Laser Therapy," "Electric Stimulation Therapy," and "Low-Level Laser Therapy." Boolean operators (AND/OR) were used to refine the search results.
3.3. Eligibility Criteria
In this review, we considered studies that explored the impact of PBM and ES on the treatment of DFUs. To identify these relevant studies, we initially assessed their titles and abstracts. After this initial screening, we thoroughly reviewed the full texts of the selected studies. For inclusion in this systematic review and meta-analysis, studies had to meet specific criteria: (1) Randomized clinical trials (RCTs), (2) original articles, (3) participants with DFUs, (4) the treatment of DFUs using PBM or ES, and (5) providing specific data on DFU healing, including initial ulcer size and final ulcer size. We excluded studies that did not meet these criteria, including those with insufficient data, a lack of quantitative reporting on initial or final ulcer size, articles without full texts, animal studies, non-original articles, pilot studies, in vitro studies, letters to editors, review articles, protocols, monographs, and theses/dissertations.
3.4. Data Extraction
In the initial phase of this study, two authors independently reviewed the titles and abstracts of all the retrieved articles. Subsequently, articles that appeared relevant were selected, and their full texts were carefully examined. Pertinent information was then extracted from these articles. Furthermore, the references of these relevant articles were investigated to identify any additional relevant sources. Any discrepancies or differences in judgment between the authors were resolved through discussion with the involvement of the corresponding author. To systematically gather and organize the information from the selected articles, an Excel spreadsheet was designed. The following details were extracted and entered into this spreadsheet: Author names, publication years, study countries, information about the intervention and control groups, initial ulcer sizes, final ulcer sizes, Wagner ulcer grade, average duration of ulcers, the number of treatment sessions, PBM/ES parameters, and the study outcomes. Additionally, a separate Excel sheet was used to assess the quality of the articles based on predefined quality assessment criteria.
3.5. Quality Assessment
The Effective Public Health Practice Project (EPHPP) tool was employed to appraise the methodology of the selected articles (29). This tool allows for the assessment of six key components.
(1) Study design: Evaluating the structure and design of the study.
(2) Withdrawals and dropouts: Examining the handling of participant withdrawals and dropouts.
(3) Data collection practices: Assessing the methods used for data collection.
(4) Selection bias: Investigating any bias in the selection of participants.
(5) Blinding in controlled trials: Examining whether blinding was appropriately implemented in controlled trials.
(6) Confounders: Analyzing the identification and management of potential confounding variables.
The EPHPP tool proves valuable for evaluating the quality of quantitative studies. Based on the aforementioned criteria, studies are categorized into one of three levels.
(1) Strong: No weak ratings in any of the six aspects.
(2) Moderate: One weak rating in any of the six aspects.
(3) Weak: Two or more weak ratings across the six aspects.
Two authors conducted the quality assessment of the selected articles. After independently completing their assessments, the results were compared, and any discrepancies between the authors were resolved through discussions with SS.
3.6. Data Analysis
Standardized mean change using change score standardization (SMCC Index) was analyzed to assess the changes in wound size before and after the intervention in the two groups. In this study, analyses related to meta-analysis were conducted using a model with random effects. To account for the heterogeneity of the studies, we used a random effects model. The random effects model in meta-analysis is used when the studies included in the analysis are assumed to have different underlying effect sizes, rather than a single common effect size. This model is particularly useful when there is significant heterogeneity among the studies, as it provides a more realistic estimate of the overall effect size by acknowledging differences between studies. In the second step, the multilevel linear mixed-effect model was used, which considered dataset variabilities and heterogeneities in the form of covariances and error terms (30-32). The values of I2, τ2, and H2 in the two groups were used to assess heterogeneity between studies. Additionally, we employed Graphical Display of Study Heterogeneity (GOSH) plots to visually inspect study heterogeneity and identify potential outliers within the included studies (33). EndNote X9, a reference management software, was utilized for resource management, and the meta-analysis was conducted using R 4.2.2 software and the metafor package.
4. Results
4.1. The Literature Search
The search results and the number of retrieved articles based on PRISMA are shown in Figure 1. The search resulted in retrieving 478 articles from five databases; after removing duplicates, 225 remained for title and abstract screening. After removing 200 articles that did not meet the inclusion criteria, the full text of 31 articles was examined. Among these, ten references were excluded due to inadequate data, lack of related intervention, or being non-randomized or pilot studies. Finally, 21 articles were included in this systematic review, and data were extracted from them. It is necessary to explain that, considering the keywords used for the search were in English, all retrieved articles had at least English titles. After checking the full text of the articles included in this systematic review, only one article had the full text in a non-English language. Considering that the authors were proficient in that non-English language, this article was included in the systematic review.
4.2. General Characteristics of the Included Studies
The general characteristics of the articles included in this systematic review and meta-analysis are given in Table 1 (Full study characteristics are provided in Appendix 1 in Supplementary File). Out of the 21 articles included in this systematic review, 20 studies were published in journals, and one study was presented at a conference. Of the 16 journals that accepted articles in this field, two journals, "Lasers in Medical Science" and "Photomedicine and Laser Surgery," had the highest acceptance rate of articles in this field with three articles each. The oldest article was published in 1992, and the most recent article was published in 2023. The highest number of published articles was related to 2017 and 2018, with four articles each. The three countries of Iran, India, and Brazil accounted for 61.9% of the published studies, with five, four, and four published articles, respectively. The therapy method was PBM in 15 studies (71.4%), ES in five studies (23.8%), and both ES and PBM in one study (4.8%). Wagner grade was not mentioned in four studies, and in most studies (n = 6, 28.6%), it was grade II. Six studies did not mention challenges or limitations. The most mentioned limitations in the studies were "small sample size" (12 studies) and "short study period" (six studies).
| Authors, Y/Country | Sample Size | Groups | Initial Size (cm2) | Final Size (cm2) | Wagner Grade | PBM/ES Parameters | Treatment Time |
|---|---|---|---|---|---|---|---|
| Zulbaran-Rojas et al, (34) 2023/USA | 16; 17 | ES + PLA | 7.4 ± 8.5; 3.1 ± 5.6 | 5.8 ± 8.0; 3.2 ± 8.7 | Not clear I-II (converted) | HVPC; 150 - 250V; ankle electrodes | Home daily for 1 hour for 4 weeks |
| Wadee et al, (35) 2021/Egypt | 25; 25; 25 | HBOT + TT + LLLT + TT + TT | 5.48 ± 0.70; 5.58 ± 0.72; 5.61± 0.75 | 0.45 ± 0.16; 0.75 ± 0.23; 5.50 ± 0.75 | II | GaAlAs laser; 850 nm; 4 J/cm2 | 3 sessions (8 min) /week for 6 weeks |
| Vitoriano et al, (36) 2019/Brazil | 6; 6 | LLLT+TT + LED+TT | 1.76 ± 1.69; 1.45 ± 1.52 | 0.36 ± 0.50; 0.64 ± 0.81 | I (converted) | 830 nm laser; 30 mW; 7 J/cm2 | 10 sessions/ 2 weeks |
| Tantawy et al, (37) 2018/Egypt | 33; 32 | LLLT + TT + infrared laser therapy + TT | 10.2 ± 5.6; 9.5 ± 4.2 | 3.7 ± 1.2; 4.1 ± 1.3 | I-II | 632 nm laser; 20 mW | 90s application/cm2 and the dose of 5 J/cm2 for 8 weeks |
| Frangez et al, (38) 2018/Slovenia | 30; 30 | LLLT + TT + PLA + TT | 13.15; 15.84 | 7.364; 10.296 | NA | Wavelengths: 625/660/850 nm; 2.4 J/cm2 | 3 sessions/week for 8 weeks (5 min) |
| Priyadarshini et al, (39) 2018/India | 50; 50 | LLLT + TT | 13.74 ± 11.88; 19.09 ± 15.03 | 3.97 ± 5.41; 18.80 ± 17.70 | I-II | 660nm laser; 4 - 8 J/cm2 | 15 day (20 min) |
| de Alencar Fonseca Santos et al, (40) 2018/Brazil | 8; 8 | LLLT + TT | 1.83 ± 1.08; 2.97 ± 1.66 | 0.32 ± 0.26; 1.63 ± 1.57 | II-III (converted) | 660 nm laser; 6 J/cm2 | 4 sessions /week for 4 weeks |
| Srilestari et al, (41) 2017/Indonesia | 18; 18 | LLLT + TT + SHAM | 4.75(0.10 - 9.94); 2.33 (0.90 - 9.88) | 0.24 (0.00 -2.54); 1.25 (0.00 - 8.11) | I-III (converted) | 630 nm laser; 100 mW; 4 J/point | 2 sessions/week for 4 weeks |
| Asadi et al, (42)2017/Iran | 13; 11 | ES + TT + PLA + TT | 4.19 ± 2.2; 3.82 ± 1.7 | 1.83 ± 1.63; 2.88 ± 1.51 | II | Cathodal DC; sensory threshold | 3 sessions/week for 4 weeks |
| Mathur et al, (21) 2017/India | 15; 15 | LLLT + TT + TT | 14.84; 13.52 | 9.30; 11.46 | I | 660 nm laser; 3 J/cm2 | 15 day (1 min) |
| El Rasheed et al, (43) 2017/India | 15; 15 | PEMF + TT + LLLT+ TT | 13.096 ± 5.93; 17.55 ± 12.1 | 5.84 ± 3.63; 2.033 ± 2.01 | II | 904 nm laser; 10 J/cm2 | 3 sessions (10 min) /week for 4 weeks |
| Hoseini Sanati et al, (44) 2016/Iran | 15; 12 | LLLT + PLA | 4.94 ± 2.91; 4.63 ± 2.34 | 1.25 ± 1.04; 3.30 ± 1.25 | II | 904 nm laser; 2 J/cm2 | 3 sessions /week for 4 weeks |
| Asadi et al, (45) 2015/Iran | 10; 10 | ES + PLA | 4.05 ± 2.01; 4.27 ± 3.2 | 1.01± 0.8; 2.6 ±1.1 | II | Cathodal DC; sensory threshold | 3 sessions/week for 4 weeks |
| Feitosa et al, (46) 2015/Brazil | 8; 8 | LLLT+ TT + TT | 7.98 ± 2.06; 2.55 ± 0.77 | 2.39 ± 1.26; 8.43 ± 1.84 | NA | 632.8 nm laser; 4 J/cm2 | 3 sessions (80s) /week for 4 weeks |
| Mohajeri-Tehrani et al, (47) 2014/Iran | 10; 10 | ESP + LA | 2.48 ± 0.97; 2.43 ± 0.39 | 1.71 ± 0.66 not clear; 2.18 ± 0.35 not clear | II | Cathodal DC; sensory threshold | 3 sessions (1 hour) /week for 4 weeks |
| Sandova Ortíz et al, (48) 2014/Colombia | 9; 10; 9 | ES + LLLT + TT | NA | NA | I-II | 685 nm laser; 30 mW / 100 pps ES | 3 sessions (45min) /week for 16 weeks or until the wound closed |
| Kajagar et al, (49) 2012/India | 34; 34 | LLLT+ TT + TT | 26.08 ± 68.31; 27.47 ± 6.03 | 15.64 ± 43.73; 24.24 ± 5.51 | I | Pulsed laser; 5 kHz; 2 - 4 J/cm2 | Daily for 15 days |
| Kaviani et al, (50) 2011/Iran | 13; 10 | LLLT + TTPLA + TT | 10.7 ± 25.7; 7.8 ± 11 | 73.7 ± 10.2 (reduction %); 47.3 ± 15.4 (reduction %) | I-II | 685 nm laser; 10 J/cm2 | 6 sessions/week |
| Minatel et al, (51) 2009/Brazil | 7; 7 | LLLT + TTPLA + TT | 11.8 ± 20.46; 3.8 ± 4.13 | NA | NA | Wavelengths: (660 + 890 nm); 3 J/cm2 | 2 sessions/week for 90 days |
| Naidu et al, (52) 2005/Malaysia | 8; 8 | LLLT + TT | 5.28; 4.21 | 1.31; 3.59 | I | He-Ne laser; 1 - 5 J/cm2 | 5 sessions/week for 6 weeks |
| Lundeberg et al, (53) 1992/Sweden | 32; 32 | ES + TTPLA + TT | 24.2 ± 12.6; 22 ± 9.6 | 39 ± 14 (remaining %); 59 ± 11 (remaining %) | NA | 80 Hz AC; muscle contraction | 14 sessions/week for 12 weeks |
Abbreviations: PBM, photobiomodulation; ES, electrical stimulation; HVPC, high voltage pulsed current; HBOT, hyperbaric oxygen therapy; TT, traditional treatment; LLLT, low-level laser therapy; DFU, diabetic foot ulcer; LED, light-emitting diodes.
a Full intervention details are provided in Supplementary Table 1.
4.3. Effects of Photobiomodulation and ES on Wound Size
Meta-analysis was used to investigate changes in wound size before and after the intervention. The characteristics of the studies included in the meta-analysis are given in Table 2.
| Groups | Number |
|---|---|
| PBM; author (y) | |
| Wadee et al. (2021) (35) | 25 |
| Hoseini Sanati et al. (2016) (44) | 15 |
| Feitosa et al. (2015) (46) | 8 |
| Kajagar et al. (2012) (49) | 34 |
| Priyadarshini et al. (2018) (39) | 50 |
| Santos et al. (2018) (16) | 8 |
| ES; author (y) | |
| Zulbaran-Rojas et al. (2023) (34) | 16 |
| Asadi et al. (2017) (42) | 13 |
| Asadi et al. (2015) (45) | 10 |
| Lundeberg et al. (1992) (53) | 32 |
| Mohajeri-Tehrani et al. (2014) (47) | 10 |
Abbreviation: PBM, photobiomodulation; ES, electrical stimulation.
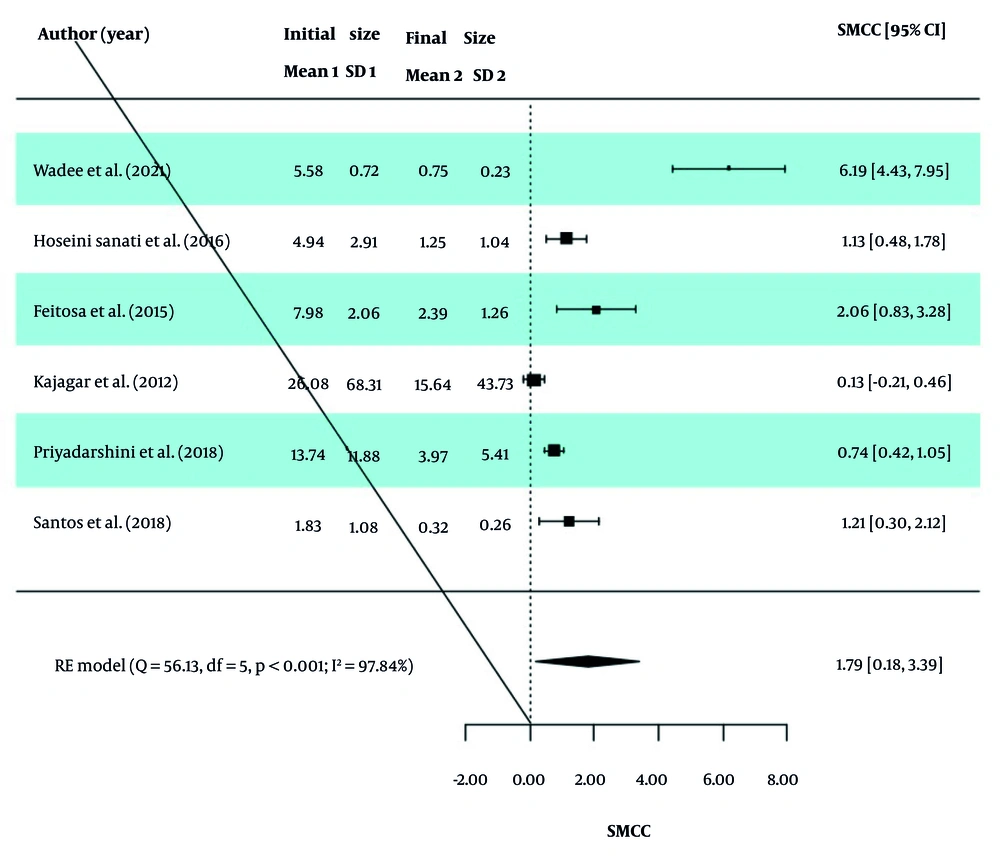
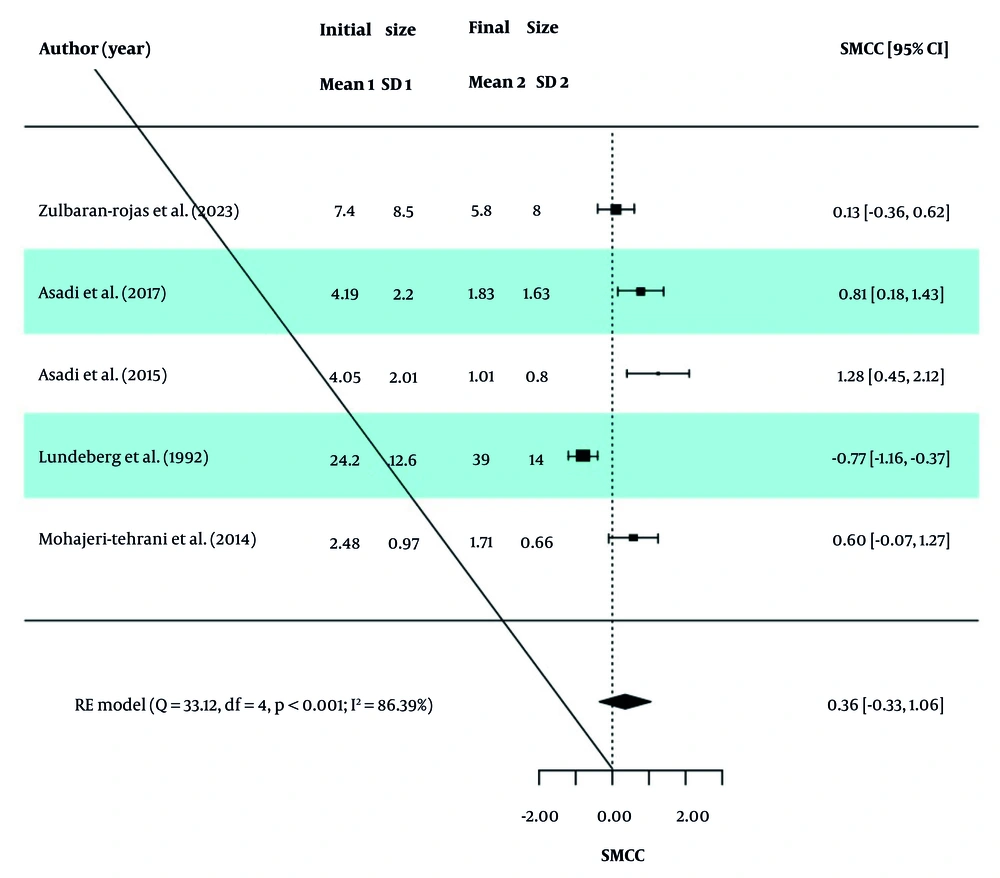
In each study, the SMCC Index was analyzed to assess the changes in wound size before and after the intervention in the two groups. The overall SMCC value, along with the homogeneity indices of the studies, is reported in Table 3. Based on the Q-test results (P < 0.0001 for both groups), significant heterogeneity was observed between the effect sizes of studies in both the PBM and ES groups. Therefore, a random-effects model (RE model) was used to calculate the effect sizes. In the PBM group, the SMCC was 1.79 (95% CI: 0.18, 3.39), indicating a statistically significant reduction in wound size after the intervention (Z statistic = 2.18, P = 0.029). Similarly, the SMCC in the ES group was 0.36 (95% CI: -0.33, 1.06), suggesting a reduction in wound size, though this was not statistically significant (Z statistic = 1.02, P = 0.308).
| Groups | SMCC (95% CI) | Z Statistics (P-Value) | Q Statistics (P-Value) | I2 | H2 | τ2 |
|---|---|---|---|---|---|---|
| PBM | 1.79 (0.18, 3.39) | 2.18 (0.029) | 56.13 (< 0.0001) | 97.84 | 46.20 | 3.78 |
| ES | 0.36 (-0.33, 1.06) | 1.02 (0.308) | 33.12 (< 0.0001) | 86.39 | 7.35 | 0.53 |
Abbreviations: SMCC, standardized mean change using change score standardization; CI, confidence interval; PBM, photobiomodulation; ES, electrical stimulation.
The values of I2, τ2, and H2 in the two groups indicate the presence of high dispersion and heterogeneity between studies, and therefore the obtained SMCC values are not valid. Also, forest diagrams for the PBM and ES groups are presented in Figures 2 and 3.
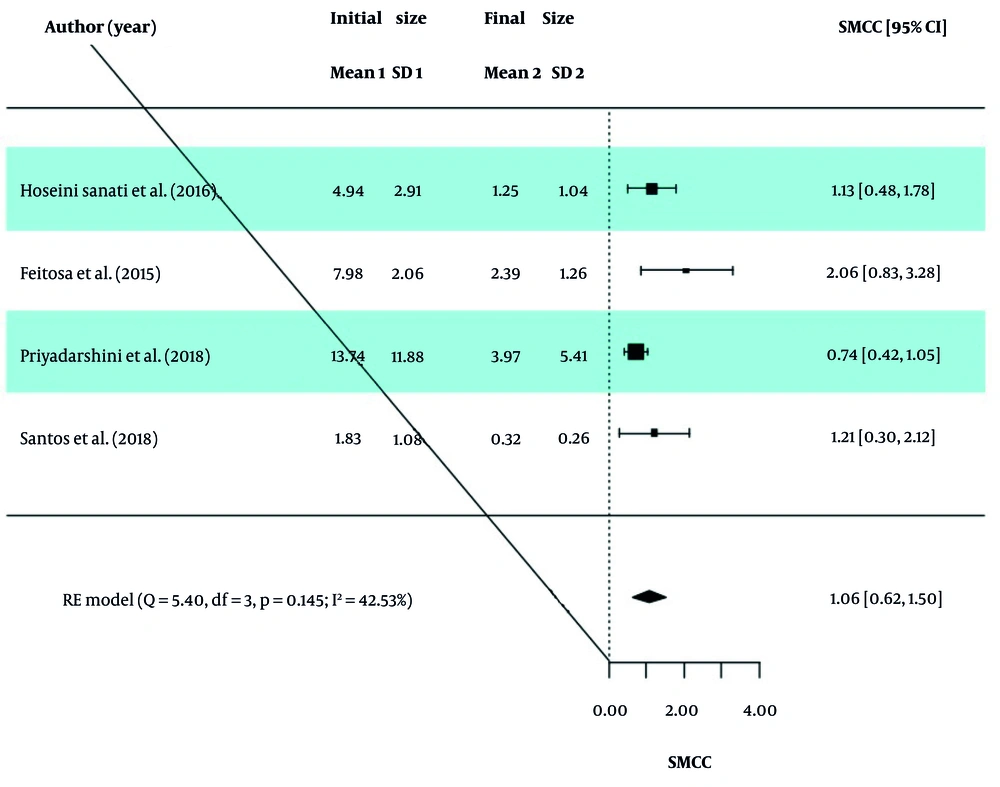
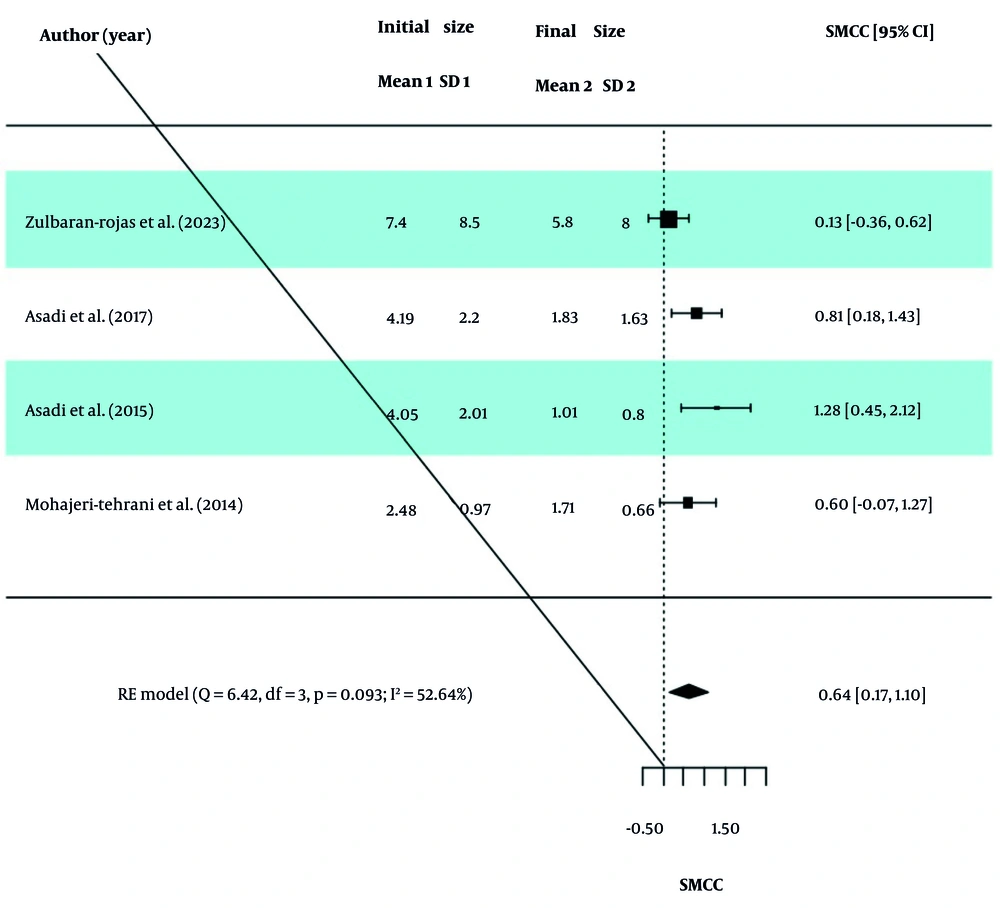
The GOSH analysis was used to check for outliers in the included studies. After removing studies 1 and 4 from the PBM group and study 4 from the ES group, the results were extracted again and reported in Table 4. The obtained values of I2, τ2, and H2 in the two groups indicate the existence of moderate dispersion between the studies, and therefore the obtained SMCC values were valid. Intervention in both the PBM and ES groups reduced the wound size, and this size change was statistically significant. Forest diagrams for the PBM and ES groups are presented in Figures 4 and 5.
| Groups | SMCC (95% CI) | Z Statistics (P-Value) | Q Statistics (P-Value) | I2 | H2 | τ2 |
|---|---|---|---|---|---|---|
| PBM | 1.06 (0.62, 1.50) | 4.69 (< 0.0001) | 5.39 (0.145) | 42.53 | 1.74 | 0.09 |
| ES | 0.64 (0.17, 1.10) | 2.67 (0.008) | 6.42 (0.093) | 52.64 | 2.11 | 0.12 |
Abbreviations: SMCC, standardized mean change using change score standardization; CI, confidence interval; PBM, photobiomodulation; ES, electrical stimulation.
The effect of the BMI variable on the wound size in the groups is reported in Table 5. In the PBM group, although the effect of the BMI variable was not significant (P = 0.937), adding this variable to the model reduced I2, τ2, and H2 values. In the ES group, the effect of the BMI variable was significant (P = 0.017), and adding this variable to the model caused a significant decrease in I2, τ2, and H2 values. To investigate the effect of time (before and after intervention), BMI, and group (PBM and ES) on the wound size mean, multilevel meta-analysis using mixed-effect models was applied.
| Groups | Coefficient (95% CI) | Z Statistics (P-Value) | I2 | H2 | τ2 |
|---|---|---|---|---|---|
| PBM | 42.37 | 1.74 | 0.05 | ||
| Intercept | 1.68 (-17.08, 20.44) | 0.17 (0.861) | |||
| BMI | -0.03 (-0.72, 0.664) | -0.08 (0.937) | |||
| ES | 0.00 | 1.00 | 0.00 | ||
| Intercept | 3.68 (1.09, 6.29) | 2.78 (0.006) | |||
| BMI | -0.11 (-0.19, -0.02) | -2.39 (0.017) |
Abbreviations: CI, confidence interval; BMI, Body Mass Index; PBM, photobiomodulation; ES, electrical stimulation.
The results of this analysis are reported in Table 6. According to the results, the wound size mean after the intervention was smaller than before the intervention, and it was statistically significant (P = 0.002). Also, the wound size mean in the PBM group was smaller than in the ES group but wasn’t statistically significant (P = 0.095).
| Variables | Coefficient (95% CI) | Z Statistics (P-Value) |
|---|---|---|
| Intercept | -5.84 (-20.48,8.98) | -0.78 (0.433) |
| Time (Final) | -3.53 (-5.79, -1.25) | -3.05 (0.002) |
| Group (PBM) | -1.78 (-3.85, 0.31) | -1.67 (0.095) |
| BMI | 0.45 (-0.11, 1.01) | 1.56 (0.118) |
Abbreviations: CI, confidence interval; BMI, Body Mass Index; PBM, photobiomodulation; ES, electrical stimulation.
4.4. Quality Assessment Results
The quality of the included studies was assessed using the EPHPP tool, which evaluates six key components: Selection bias, study design, confounders, blinding, data collection, and drop-outs. Each component was rated as 1 = strong, 2 = moderate, or 3 = weak, and an overall global rating was assigned to each study. The detailed results of the quality assessment are presented in Table 7.
| Studies | Selection Bias | Study Design | Confounders | Blinding | Data Collection | Drop-outs | Global Rating b |
|---|---|---|---|---|---|---|---|
| Zulbaran-Rojas et al, (34) 2023/USA | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Wadee et al, (35) 2021/Egypt | 3 | 1 | 1 | 2 | 1 | 1 | 2 |
| Vitoriano et al, (36) 2019/Brazil | 2 | 1 | 2 | 3 | 1 | 1 | 2 |
| Tantawy et al, (37) 2018/Egypt | 3 | 1 | 1 | 2 | 1 | 1 | 2 |
| de Alencar Fonseca Santos et al, (40) 2018/Brazil | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Priyadarshini et al, (39) 2018/India | 1 | 1 | 1 | 3 | 1 | 1 | 2 |
| Frangez et al, (38) 2018/Slovenia | 2 | 1 | 1 | 1 | 1 | 3 | 2 |
| Srilestari et al, (41) 2017/Indonesia | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| El Rasheed et al, (43) 2017/India | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Mathur et al, (21) 2017/India | 2 | 1 | 1 | 3 | 1 | 1 | 2 |
| Asadi et al, (42) 2017/Iran | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| Hoseini Sanati et al, (44) 2016/Iran | 2 | 1 | 3 | 3 | 1 | 2 | 3 |
| Feitosa et al, (46) 2015/Brazil | 3 | 1 | 1 | 2 | 1 | 1 | 2 |
| Asadi et al, (45) 2015/Iran | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| Sandoval Ortíz et al, (48) 2014/Colombia | 1 | 1 | 1 | 1 | 1 | 3 | 2 |
| Mohajeri-Tehrani et al, (47) 2014/Iran | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Kajagar et al, (49) 2012/India | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Kaviani et al, (50) 2011/Iran | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Minatel et al, (51) 2009/Brazil | 3 | 1 | 3 | 1 | 1 | 3 | 3 |
| Naidu et al, (52) 2005/Malaysia | 2 | 1 | 3 | 2 | 1 | 1 | 2 |
| Lundeberg et al, (53) 1992/Sweden | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
a Quality ratings are based on the Effective Public Health Practice Project (EPHPP) tool.
b The Global Rating is an overall assessment of study quality based on the EPHPP tool.
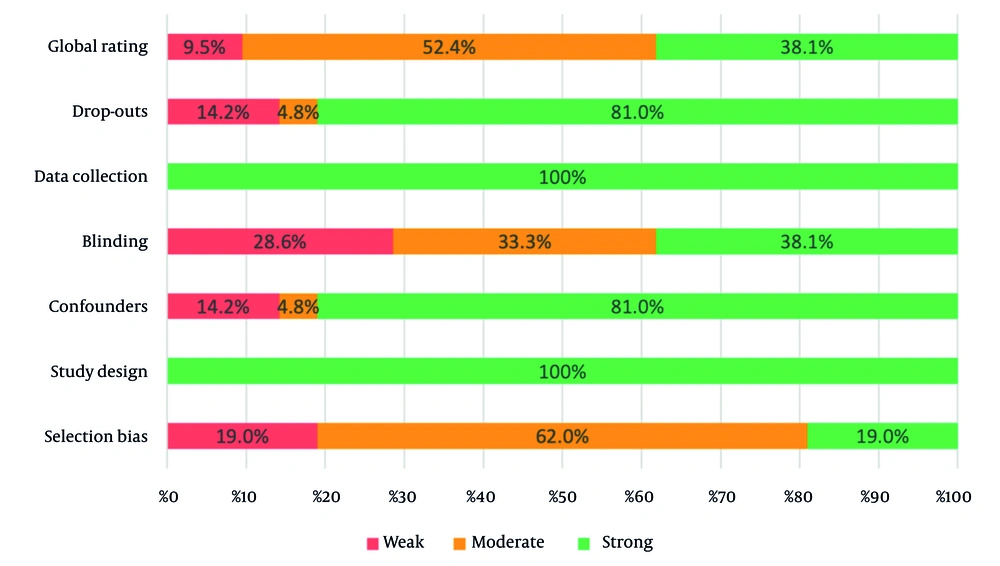
The results showed that 100% of the articles included in this systematic review were strong in terms of data collection and study design. However, 28.6% of the articles were classified as weak in terms of blinding. Global ratings also showed that 52.4% of the articles were moderate and 38.1% were strong. These findings are summarized in Figure 6.
5. Discussion
5.1. Summary of Findings
This study aimed to compare the effects of PBM and ES on the healing of DFUs. An extensive literature search was conducted across five reputable databases, initially yielding 478 articles. After screening, only 21 studies met the predefined inclusion criteria and were included in this review, highlighting the rigorous methodology utilized. Our analysis found that PBM was the predominant therapeutic intervention, utilized in 15 studies, representing 71.4% of the total. ES was employed in five studies, accounting for 23.8%. One study, comprising 4.8% of the sample, used both PBM and ES. The peak in publications in 2017 and 2018 may reflect a surge in research interest or critical developments during this period. Further investigation into the historical events and breakthroughs in those years that stimulated research output would provide insight. Tracking the progression of research historically can identify emerging areas of focus. The geographical concentration of studies in Iran, India, and Brazil suggests that these countries have substantially contributed to the field. Further investigation into the research ecosystems and collaborations in these regions can shed light on the reasons behind their high research output. This could include examining government funding initiatives, academic institutions, and partnerships with international researchers.
5.2. Efficacy of Photobiomodulation and Electrical Stimulation
The meta-analysis demonstrated that both PBM and ES significantly reduced DFU size compared to baseline. However, there was no statistically significant difference between the treatment effects of PBM and ES for decreasing ulcer area. This indicates that neither modality showed superior efficacy over the other. It should be noted, however, that PBM demonstrated a more significant effect size and standardized mean change than ES. Although this larger effect was not statistically significant, it may suggest potentially enhanced efficacy of PBM over ES. The PBM can impact various biological pathways linked to wound healing by enhancing cellular activity, improving blood circulation, and boosting growth factor levels, potentially leading to faster reductions in ulcer size (54). However, additional high-quality comparative controlled trials with larger sample sizes are required to further compare the effects of PBM and ES on reducing DFU size over time. The current findings, while promising, must be interpreted cautiously due to heterogeneity in study designs and intervention protocols across the included trials. Nevertheless, the current analysis provides evidence supporting both modalities as effective adjuvant therapies that should be considered along with standard care to enhance wound closure in this population.
5.3. Comparison with Previous Studies
The results of this study align with previous research showing the benefits of LLLT and ES for treating DFUs. A recent systematic review and meta-analysis by Santos et al. found that LLLT significantly reduced ulcer size and promoted greater healing rates than control treatments, with a 22.96% greater reduction in ulcer size with LLLT (16). Our findings on the efficacy of ES are supported by a recent meta-analysis by Chen et al. on ES for DFUs. In their analysis of seven randomized controlled trials with 274 patients, ES as an adjunct to standard wound care resulted in a significantly greater decrease in ulcer area at four weeks (standardized mean difference 1.09, 95% CI 0.62 - 1.57, P < 0.001) and a higher ulcer healing rate at 12 weeks (risk difference 0.19, 95% CI 0.06 - 0.32, P = 0.005) compared to standard wound care alone (55).
A previous study by Sandoval Ortiz et al. did not demonstrate additional effects of LLLT or high voltage pulsed current (HVPC) compared to standard wound care alone on the healing of DFUs (48). Their randomized controlled trial found no significant differences between the LLLT, HVPC, and control groups in ulcer healing rates, nerve conduction studies, protective sensation, or quality of life after 16 weeks of treatment. These negative results contradict our findings, which show improved diabetic ulcer healing with LLLT and ES. However, Sandoval Ortiz et al. had a small sample size (n = 28), which may have limited the power to detect group differences. They recommend more extensive trials to be conducted. The advanced neuropathy state of their patients (mean 11 years diabetes duration) may have also reduced treatment effects on the assessed neurological outcomes. Overall, this well-conducted study provides substantial evidence that LLLT and HVPC may not provide added benefit over standard care in treating diabetic ulcers, particularly in those with severe neuropathy. However, Sandoval Ortiz et al. did not report the initial and final ulcer sizes, precluding the inclusion of their data in a meta-analysis.
5.4. Implications for Clinical Practice
We observed a significant decrease in the size of DFUs when either PBM or ES was used in conjunction with standard care. This suggests that employing these therapies alongside conventional treatment enhances wound area reduction. There was no statistically significant difference between the treatment effects of PBM and ES for decreasing ulcer size. The side effect profile and risks should be considered when selecting between modalities. None of the studies reported adverse events with PBM treatment, likely due to low power densities avoiding tissue damage. In contrast, ES carries risks of skin irritation or burns with improper parameters. Additionally, PBM typically requires shorter treatment sessions than ES. Therefore, the improved safety profile and shorter treatment durations of PBM should be considered when choosing adjuvant therapies with standard care to improve wound closure.
Our meta-analysis comprehensively assessed the impact of BMI on wound size reduction across PBM and ES treatment groups. The findings revealed intriguing distinctions in treatment efficacy based on patients' BMI. For PBM, our analysis demonstrated that BMI did not significantly affect ulcer area reduction. This suggests that PBM demonstrates consistent therapeutic efficacy across different body weight categories. In contrast, ES exhibited a significant BMI effect, indicating reduced treatment efficacy specifically in obese diabetic patients.
The differential impact of BMI on these therapies highlights the importance of considering patient body composition when selecting treatment protocols. For ES, the significant BMI influence suggests that clinicians should carefully stratify patients and potentially develop tailored interventions for individuals with higher body weight. Supporting this observation, a study by Doheny et al. investigated the impact of subcutaneous fat thickness on ES, revealing that increased fat thickness reduced activation function and necessitated higher electrical currents for effective stimulation (56). This finding aligns closely with our research, where we observed correlations between slower wound healing in diabetic patients with higher BMI. Our results suggest that while PBM appears to be a more universally applicable adjuvant therapy for DFUs, ES may require more nuanced, patient-specific approaches. Further research is warranted to explore the underlying mechanisms driving the differential impact of BMI on these therapies and to optimize treatment protocols based on individual patient characteristics.
Individual factors such as age, sex, duration of diabetes, and neuropathy can negatively influence DFU healing (10). While these variables are known to affect wound healing, they were not always consistently controlled for in the studies included in this meta-analysis. We acknowledge that variables such as age, sex, and neuropathy were not fully addressed in every study. In the cases where these factors were reported, we integrated them as much as possible into our analysis.
The PBM and ES encounter significant challenges for real-world adoption, such as (1) high equipment costs (especially for PBM); (2) the requirement for specialized staff training; and (3) demanding treatment schedules that can impact patient adherence. Although PBM offers safety benefits, ES might present a more cost-efficient option. Future studies should tackle these obstacles by exploring subsidized programs or streamlined protocols.
5.5. Characteristics of Interventions
5.5.1. Photobiomodulation
Based on the included RCTs in our work, the PBM parameters for DFU were as follows: Wavelength of 630 - 904 nm, power density of 20 - 300 mW, and dosage of 1 - 6 J/cm2, with an irradiation time of 30 - 60 seconds at each point at a distance of 1 cm away from the wound, and at least 3 times a week for 4 - 6 weeks.
5.5.2. Electrical Stimulation
The parameters used in most trials for ES include the HVPC and DC with negative polarity, the intensity of sensory threshold, 30 - 60 minutes per day, 3 - 5 sessions per week, and electrodes placed around the wound. Yet, further evidence from higher-quality RCTs is imperative to determine the most appropriate parameters for healing DFUs.
5.6. Limitations and Potential Biases
This systematic review and meta-analysis have several important limitations that should be considered when interpreting the results. First, many of the included studies had fewer than 30 participants, which may limit the generalizability of the findings and increase the risk of random error. Additionally, many studies had short follow-up periods, often around four weeks, which provides only a snapshot of the ulcer healing trajectory and limits the ability to assess the long-term sustainability of treatment effects. Another limitation is the heterogeneity of the included studies in terms of intervention parameters, such as wavelength, power density, and treatment duration for PBM, as well as waveform, intensity, and electrode placement for ES. Finally, the quality assessment of the included studies revealed that 28.6% of the studies were weak in terms of blinding, which may have introduced performance or detection bias. These limitations underscore the need for larger, more standardized trials with longer follow-up periods to confirm these preliminary findings.
5.7. Future Research Directions
Given the limitations of current evidence, future RCTs should prioritize larger sample sizes, standardized protocols, and longer follow-up periods to validate these preliminary findings. These studies would provide more definitive, high-quality evidence regarding the impact of PBM and ES on reducing diabetic ulcer size over time. By incorporating these elements, researchers can enhance our understanding of these treatments and their long-term effects on DFUs.
6. Conclusions
This systematic review and meta-analysis found that both PBM and ES are effective adjuvant therapies for enhancing DFU healing when combined with standard care. Although PBM showed a larger effect size, the difference between the two treatments was not statistically significant. Overall, healthcare providers may consider incorporating PBM or ES to improve DFU healing. Given this, treatment selection should be guided by practical considerations such as equipment availability, cost, treatment duration, ease of use, side effect profile, and patient preference. Notably, most included studies (6 of 11) focused on Wagner grade II ulcers, indicating that these findings are generalizable primarily to moderate-severity DFUs. Future studies should investigate the efficacy of these treatments across different Wagner grades to further establish their applicability in varying ulcer severities.