1. Background
"First discovered in Wuhan, China, in late 2019, a novel pneumonia, later named COVID-19 by the World Health Organization, was caused by the SARS-CoV-2 virus (1). This disease can lead to severe respiratory failure syndrome and primarily spreads through contact with infected individuals via the inhalation of respiratory droplets and aerosols (2). The fatality rate for COVID-19 was approximately 2.2% (3), and its manifestations spanned from asymptomatic infection to acute respiratory failure."
"In this retrospective study, a substantial number of patients were observed in pre-acute stages. Hospitalized patients exhibited a 17 - 29% probability of experiencing respiratory failure. The predominant symptoms associated with the condition were fever, cough, and fatigue (4, 5). While the primary impact of COVID-19 is on the respiratory system, it is also capable of inducing dysfunction in other organ systems, such as the cardiovascular, renal, and neurological systems, thereby contributing to patient demise. Additionally, the clinical progression of SARS-CoV-2 infection has been characterized by varied outcomes (6, 7). Although the COVID-19 pandemic has been largely managed for more than a year, the disease persists as a significant global health concern."
Electrolytes are vital for numerous biological processes, including maintaining cellular function and enabling nerve and muscle activity. Essential electrolytes like sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), chloride, phosphate, and bicarbonate are obtained through diet and fluids. Emerging evidence suggests that COVID-19 infection frequently leads to electrolyte disturbances, particularly involving Na, K, and Ca, with more severe cases often presenting with hypokalemia (8, 9). These imbalances are widely recognized as common in COVID-19 patients and can significantly impact disease severity and progression. Hyponatremia (low Na) and hypokalemia (low K) are frequently associated with worse outcomes, including increased ICU admissions and mortality, possibly due to factors such as syndrome of inappropriate antidiuretic hormone secretion (SIADH), inflammatory responses, direct viral effects on renal tubular cells, or gastrointestinal losses (10-13). However, some studies present conflicting findings regarding the direction of these associations, highlighting the need for further research to clarify these discrepancies and better understand the underlying pathophysiological mechanisms.
The presence of electrolyte abnormalities is believed to significantly influence the trajectory of COVID-19. A thorough understanding and monitoring of these imbalances are crucial for improving clinical management, accurately predicting prognosis, and identifying potential therapeutic targets. These changes in body electrolyte levels in COVID-19 patients are not merely incidental findings. Instead, they are deeply intertwined with the severe pathophysiological processes of the disease. This includes direct viral damage to organs like the kidneys, dysregulation of the Renin-Angiotensin System (RAS), and the destructive effects of the cytokine storm. These factors collectively contribute to multi-organ dysfunction and, ultimately, increased mortality (14-16). While some studies report hypokalemia and hyponatremia as markers of severity (17-19), other research has found elevated Na and K levels in non-survivors (20), underscoring the complexity and the critical need for personalized monitoring (21).
2. Objectives
This study, therefore, aims to investigate the association between serum electrolyte levels at hospitalization and patient mortality in COVID-19.
3. Methods
3.1. Study Design
This retrospective case-control study was conducted at Kowsar Hospital, affiliated with Semnan University of Medical Sciences in Semnan, Iran, from July 1, 2021, to June 31, 2022, during the local epidemic of the COVID-19 Delta variant. This study involved a census of all hospitalized patients during the designated epidemic period, whose medical records were subsequently reviewed. To ensure data reliability, two independent researchers extracted all relevant information from the medical records. Any discrepancies were resolved through discussion and consensus, or by consultation with a third senior researcher.
Patients were excluded if they had pre-existing conditions known to significantly or acutely confound electrolyte levels at admission, including chronic kidney disease (CKD) requiring dialysis, thyroid or parathyroid diseases, uncontrolled diabetes (defined as HbA1c > 9% or random blood glucose > 300 mg/dL upon admission), or a Body Mass Index (BMI) exceeding 40 kg/m2. Patients were also excluded if they were receiving medications known to acutely alter electrolyte balance (e.g., high-dose diuretics or specific intravenous electrolyte infusions) at the time of admission.
Patient outcomes (death or discharge) were ascertained from their medical records, and patients were then stratified into two distinct groups based on these outcomes: Deceased patients as the case group and survivors as the control group.
3.2. Data Collection
Demographic data (age, gender, underlying comorbidities), clinical characteristics, and initial laboratory results recorded at admission were extracted from patient medical records. Standard reference ranges used by the hospital laboratory for serum electrolytes were as follows: The Na (135 - 145 mEq/L), K (3.5 - 5.0 mEq/L), Ca (8.5 - 10.2 mg/dL), and Mg (1.7 - 2.3 mg/dL). The initial 5-mL blood samples, collected as part of routine clinical management, were used for serum electrolyte analysis (Na, K, Ca, and Mg). These samples were collected by hospital staff following standard clinical protocols, and tests were conducted using a standardized diagnostic kit in the designated hospital laboratory, ensuring consistency in measurement protocols.
3.3. Data Analysis
Qualitative variables are presented as percentages and ratios, while quantitative variables are expressed as means and standard deviations. Statistical analysis involved independent t-tests and chi-square tests. Data analysis was performed in the SPSS statistical program version 24 at a significance level of less than 0.05.
4. Results
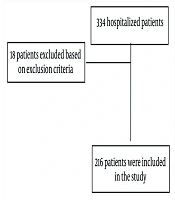
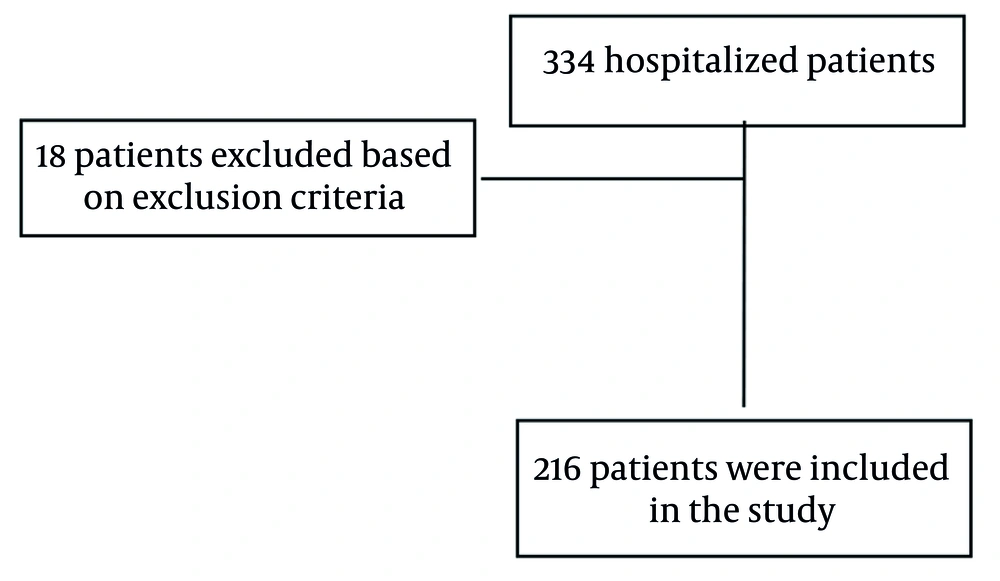
During the study period, 334 patients were admitted to our center. Of these, 18 were excluded based on exclusion criteria, resulting in 316 individuals being included in the analysis. Following follow-up, 66 patients died, and 250 were discharged (Figure 1).
Table 1 presents the demographic and baseline characteristics of the patients. A statistically significant difference in age was observed between expired and discharged patients (P < 0.05).
Significant differences between the two groups were observed only in the levels of Na, K, and albumin, following the evaluation of four main electrolytes and albumin. Among patients who died of COVID-19, Na and K levels were elevated, while albumin levels were reduced, compared to the other group (Table 2).
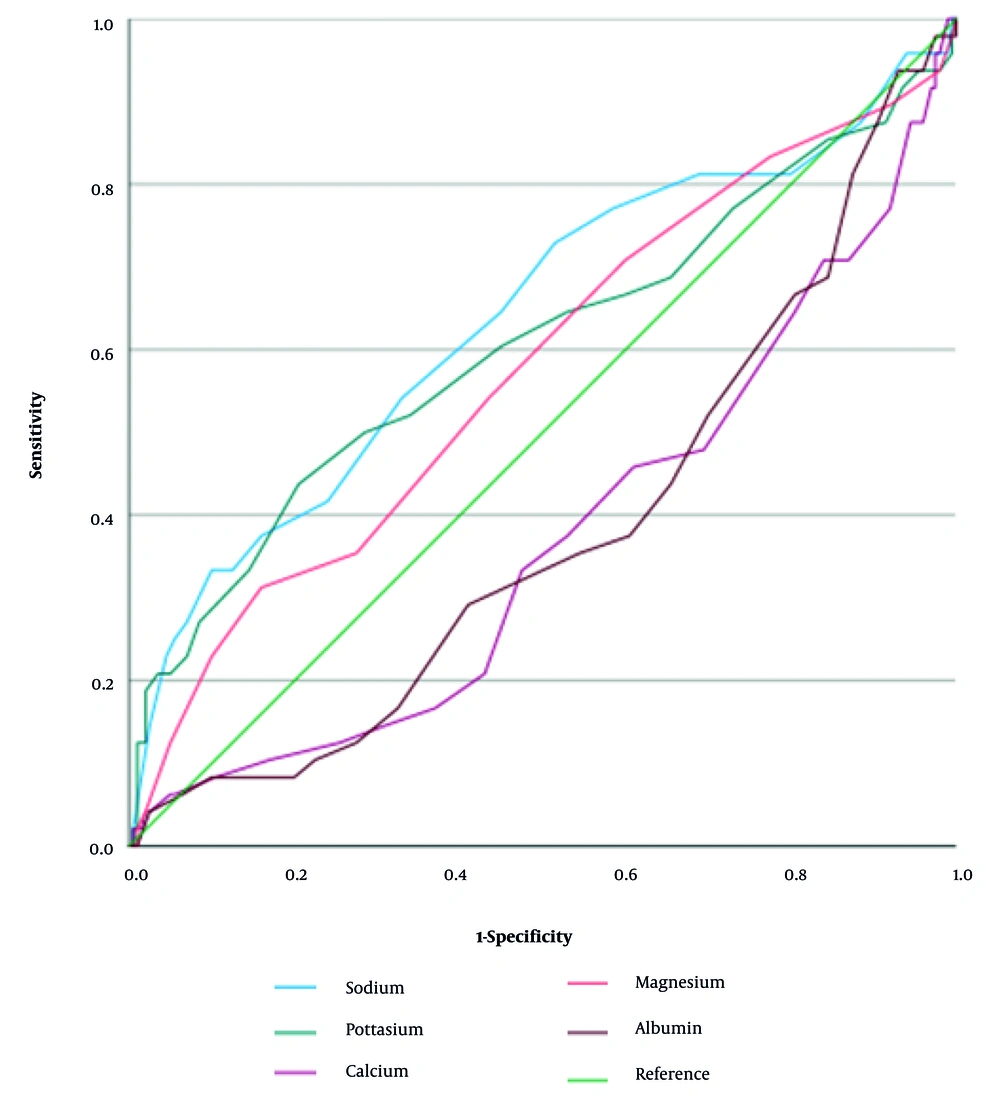
To assess the predictive value of serum electrolyte and albumin levels for patient mortality, Receiver Operating Characteristic (ROC) curve analysis was performed, and the area under the curve (AUC) was calculated for each biomarker. The AUC values were 0.637 for Na, 0.605 for K, 0.370 for Ca, 0.576 for Mg, and 0.382 for albumin. These results indicated that Na and K exhibited moderate predictive power, while Mg showed only poor predictive power. The Ca and albumin lacked significant predictive value, with AUC values below 0.5 suggesting no better performance than chance (Figure 2).
Given the significant difference in mean age observed between deceased and recovered patients at baseline, multivariate logistic regression analysis was conducted to adjust for the effect of age and other potential confounders on patient outcome. This analysis revealed that only age and length of hospital stay were significantly associated with mortality. Other variables, including underlying diseases, BMI, and serum levels of electrolytes (Na, K, Ca, and Mg) and albumin, were not significantly associated with disease outcome. Specifically, each standard deviation increase in length of hospital stay was associated with a 1.130-fold increase in the odds of mortality (OR = 1.130, 95% CI: 1.022 - 1.250, P = 0.017). Similarly, each standard deviation increase in age was associated with a 1.033-fold increase in the odds of mortality (OR = 1.033, 95% CI: 1.001 - 1.066, P = 0.040).
5. Discussion
The emergence of COVID-19 rapidly escalated into a global health crisis, leading to a significant number of fatalities worldwide. Recognizing the critical role of serum electrolytes in maintaining proper physiological function, this study aimed to determine the association between mortality and serum levels of Na, K, Ca, and Mg in COVID-19 patients. Our investigation demonstrated a robust relationship between age and patient outcomes (mortality or discharge). However, it is crucial to note that after adjusting for confounders in multivariate logistic regression, only age and length of hospital stay remained significantly associated with mortality. While initial analyses (e.g., AUC) suggested associations with serum Na and K levels, these associations did not persist as independent predictors in the multivariate model. Conversely, no statistically significant independent association was observed for BMI, serum Ca, Mg, or albumin levels with patient outcome.
Specifically, this study found a statistically significant relationship between patient age and outcomes, indicating that mortality rates increase with age. This finding aligns with previous research by Demombynes (22) and Caramelo et al. (23), who consistently reported higher mortality rates among older COVID-19 patients. Numerous studies evaluating the relationship between age and mortality have consistently yielded similar conclusions. It is hypothesized that aging, often accompanied by an increase in underlying comorbidities and a decline in immune function (immunosenescence), contributes to increased mortality in older individuals. For instance, Alizadeh et al. demonstrated that in older adults with COVID-19, a higher neutrophil-to-lymphocyte ratio (NLR) correlates with both increased mortality and more severe illness (24). Therefore, based on both prior research and the current investigation, advanced age is an independent risk factor for COVID-19 mortality.
Our study found no statistically significant relationship between gender and mortality in COVID-19 patients, despite observing a non-significant trend towards a higher death rate in males compared to females. This outcome contrasts with several published studies, including that by Caramelo et al. (23) and a meta-analysis by Nasiri et al. (2020) (25), which reported a heightened mortality risk for males. This divergence in findings could be explained by differences in our study's statistical population, sample size, or specific regional patient characteristics.
Beyond these methodological considerations, biological and behavioral factors are often cited for observed gender disparities in COVID-19 mortality. Biologically, females are known to mount a more robust immune response, evidenced by greater anti-COVID-19 IgG antibody production (26). From a behavioral perspective, males typically exhibit a higher prevalence of risky behaviors, such as smoking and alcohol consumption. The World Health Organization, for instance, estimates that roughly 40% of men globally smoke, whereas only about 9% of females are smokers (27). Additionally, females generally demonstrate a greater inclination towards health consciousness and preventative practices (28).
Our initial analysis (Table 2, Figure 2) revealed a significant difference in serum Na and K levels between deceased and discharged patients, with higher Na and K levels in non-survivors. However, importantly, our multivariate logistic regression analysis demonstrated that these differences were not independently associated with mortality after adjusting for age and length of hospital stay. This specific finding, particularly concerning the elevated levels of Na and K in non-survivors, appears to contradict some previous reports. For instance, Lippi et al. (8) associated hypokalemia, hyponatremia, and hypocalcemia with severe COVID-19, while Alamdari (28) found that deficiencies in Mg and K correlated with increased mortality. Furthermore, Wang et al. (9) reported significantly lower K levels in patients with severe COVID-19.
Despite these discrepancies and our multivariate findings, electrolyte imbalances, especially involving Na and K, are widely recognized as common occurrences in COVID-19 patients and can influence disease severity and progression. Generally, hyponatremia (low Na, typically < 135 mmol/L) is frequently linked to increased disease severity, higher ICU admission rates, and mortality. Its causes can include SIADH or inflammatory responses. Hypernatremia (high sodium, typically > 145 mmol/L), though less common at admission, can develop in critical cases and is associated with adverse outcomes due to dehydration or fluid imbalance. Similarly, hypokalemia (low K, typically < 3.5 mmol/L) is prevalent and often associated with severe disease and higher mortality. Proposed mechanisms include renal tubular dysfunction due to direct viral effects, increased angiotensin II (Ang II) activity, or gastrointestinal losses.
While many studies associate low levels of Na and K with worse outcomes, the varying directions of association observed across studies highlight the influence of differences in study design, patient cohorts, sample size, or the timing of electrolyte measurement. Nevertheless, monitoring Na and K levels remains crucial as they reflect underlying physiological disturbances and can impact patient outcomes (10-13).
The observed deviations in serum electrolyte levels in COVID-19 patients are hypothesized to be influenced by the activation of the RAS via a modified, downregulated angiotensin-converting enzyme 2 (ACE2). During viral proliferation, ACE2 is utilized, and its expression is downregulated (29). This downregulation abrogates ACE2's antagonistic activity against the RAS pathway, shifting the balance towards the ACE/Ang II pathway (30). The Ang II, in turn, activates the angiotensin-2 type 1 receptor, which is responsible for the kidneys' reuptake of Na and water. Furthermore, elevated aldosterone levels lead to a dramatic increase in urinary K excretion (31, 32).
SARS-CoV-2 can directly attack renal tubular cells, resulting in renal tubular dysfunction (33). Complex proximal tubular epithelial cells and renal podocyte cells are known to express ACE2 and TMPRSS2, making them vulnerable to viral attack (34). Transmission electron microscopy has also revealed viral particles within renal proximal tubular cells and renal podocyte cells (35). Beyond renal involvement, gastrointestinal manifestations can also contribute to electrolyte imbalances (33).
The discrepancies between our findings and some prior studies regarding electrolyte levels might be attributed to differences in sample size, patient population characteristics, or assay methodologies. Renal damage caused by the virus can lead to electrolyte imbalances. Moreover, in patients recovering from COVID-19, vital organs such as the kidneys and heart may still be affected, ultimately contributing to mortality. It can be argued that electrolyte imbalances, potentially stemming from renal damage, represent one of several sequelae of COVID-19 known to be a mortality factor.
This research was conducted during the COVID-19 Delta variant outbreak in Semnan, Iran, when most admitted patients were presumed to be infected with this variant. However, a limitation of this study was the inability to conclusively identify the variant using molecular approaches due to restricted laboratory equipment. It is hypothesized that numerous known and unknown variables may influence the mortality rate of COVID-19 patients. Investigating all these factors, particularly the type and severity of pulmonary involvement, hospital facilities and services, and the regulation of metabolic diseases, was beyond the scope of this study. Therefore, further studies in larger populations are recommended.
This study faced several inherent limitations due to its retrospective design. For instance, electrolyte levels were only measured at admission; their dynamic changes throughout hospitalization until discharge or death could not be assessed, as serial measurements were not consistently available for all patients. This limitation should be addressed in future prospective studies, which could also explore the clinical utility of longitudinal electrolyte monitoring.
Furthermore, while our multivariate regression model included 'underlying diseases' as a composite variable, the retrospective nature of our data limited our ability to conduct a more granular analysis of specific comorbidities such as diabetes, heart failure, and chronic renal disease. Detailed and consistent information regarding the severity and specific subtypes of these conditions, and other relevant renal function markers (e.g., creatinine, BUN, or eGFR), was not uniformly available in the medical records for all patients. Future prospective studies should aim to collect comprehensive data on these specific comorbidities and renal function indicators to provide a more nuanced understanding of their influence on patient outcomes.
5.1. Conclusions
The results of this study highlight the well-documented relationship between increasing age and higher mortality in COVID-19 patients, confirming advanced age as an independent risk factor. Additionally, longer hospital stays were independently associated with increased mortality. While initial analyses (e.g., AUC, unadjusted comparisons) indicated a correlation between elevated serum Na and K levels at hospitalization and a greater death rate, these associations did not remain statistically significant after adjusting for age and length of hospital stay in multivariate analysis. Future investigations in broader and more diverse populations are advisable to further explore these complex relationships and confirm observations.