1. Background
There is an increasing concern over environmental pollutions because during the recent decades, human activities have led to a considerable increase in air pollution. The control and treatment of air pollutants are among the most important environmental challenges today. Therefore, the need to treat and control air pollution and serious health and environmental concerns has emerged these years. Air is a vital element to many living things. People may survive for two weeks without food and for two days without water; however, without air, a person may only survive for two minutes. In daily life, an average person consumes approximately 1 kg of food, 2 kg of water, and 20 kg of air (1). Industrial growth has led to emission of different kinds of pollutants into the air (2, 3). The need to control and air pollution treatment has become an emerging environmental and health concern for the past forty years (4). As information about the hazardous effects of contaminants increases, industry is facing new regulations for contaminated air discharged from different activities (5). It is important to note that the malodor caused by H2S has been an environmental and public nuisance in the recent years. Of particular significance are the malodors emanating from the food processing industry, common effluent treatment systems, night soil and solid waste processing plants. In particular, inhalation of low concentrations of hydrogen sulfide can cause headache, dizziness, nausea, cramps, staggering, excitability, and drowsiness. In actual situations such as industrial emissions, the strong odor threshold and vapor density cause them to travel several kilometers from the emission site (6). In order to solve environmental problems more effectively, researchers need to focus on design and optimization of the treatment processes for environmental pollution (7). Biofiltration is one of the earliest biological processes developed for the removal of gaseous compounds. This system is becoming more and more popular because it is a green technology that neither uses chemicals nor produces wastes that are potentially dangerous for the environment. This process is essentially based on the ability of microorganisms to transform organic and inorganic pollutants into less toxic and odorless compounds (8).
2. Objectives
The amount of bacterial population in the packing material of the biologic filter is very important to the treatment of air pollution (9). It is important to note that pH is a certain parameter in any biofiltration system application (10, 11). Therefore, the main goal of this study was to investigate the changes in microbial population and pH value in the two new packing materials, namely, sewage sludge (SS) and mixture of SS with rice husk silica (MSSRHS) to remove H2S pollution.
3. Materials and Methods
3.1. Preparation of Packing Materials
This work was a lab-scale study that was performed in the laboratory of Environmental Studies Faculty at the University Putra Malaysia. Two packing materials, namely, SS and MSSRHS were used as packing material to remove H2S. The SS with mixed microbial culture was collected from a sewage treatment plant (Putrajaya STP 2) in Malaysia. The rice husk silica (RHS) was prepared according to the method proposed by Jamwal and Mantri (12). The rice husk was first washed with tap water to remove dirt and other contaminants. It was then dried in an oven at about 110°C for about 24 hours. The husk was then subjected to acid leaching by refluxing it in 3% (v/v) chloridric acid and 10% (v/v) sulfuric acid for two hours at a ratio of 50 g husk/L. After acid leaching, the husk was thoroughly washed with distilled water and then dried in an air oven at 100°C. An incineration temperature of 800°C was chosen to incinerate the cleaned rice husk inside a muffle furnace for four hours in static air. Finally, RHS was prepared with a length of 0.5 to 1.5 mm. The SS was mixed with RHS (50:50 volume) to be used as a packing material. Some important properties of the dried SS used in this study are shown in Table 1.
3.2. Operation of the System
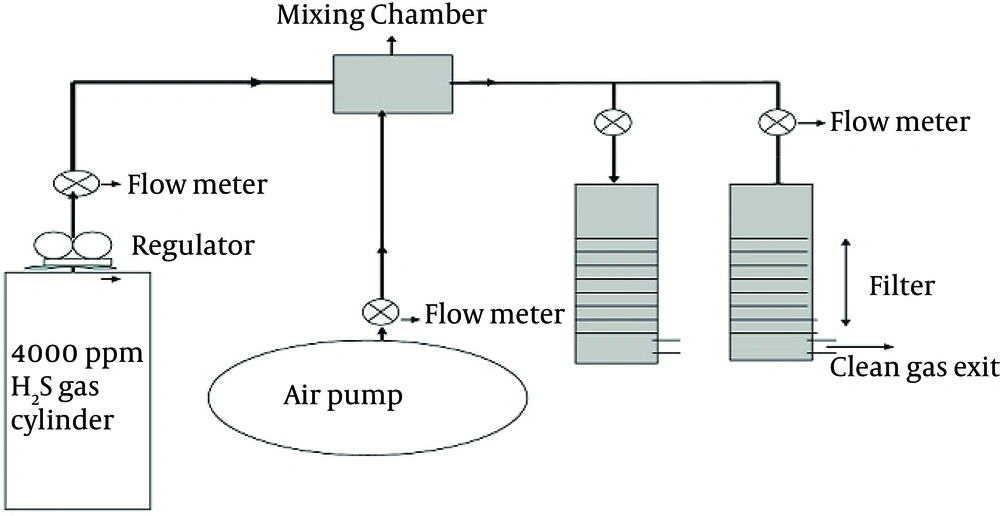
Two filters were made from PVC, with 50 cm in height and 7.5 cm in filters internal diameter (packing material volume of the filters were 1.0 L). A porous plate was used in the bottom of each filter’s bed to support the packing materials. The schematic flow diagram of this pilot is shown in Figure 1.
This research was performed in two stages. In the first stage, the performance of the filters was evaluated during the start-up period at a constant empty bed residence time (EBRT) of 60 seconds and different inlet concentrations of H2S ranging from 10 to 300 ppm. According to the study by Jeong et al. in order to adapt the microorganisms in the filters, the tested H2S gas was introduced in the filters at low concentration of 10 ppm for one week (6). After the adaptation period, H2S concentration at the inlet was increased in weekly increments. In the second stage, the flow rate (L/min) was variable with different EBRTs ranging from 30 to 90 seconds (EBRTs of 30, 45, 60, 75, and 90 seconds) and the concentration of H2S was fixed at 300 ppm.
In this study, the system operated under different conditions of two parameters, i.e. different inlet gas concentration and different inlet gas flow rate. The inlet and outlet concentrations of H2S were measured using H2S detector (model ppb RAE 3000, the United States). Removal efficiency (RE) and elimination capacity (EC) were used as performance of filters according to the following equations:
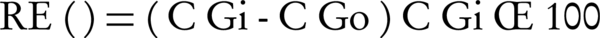
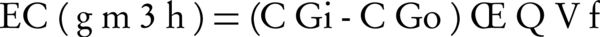
Where Q is the gas flow rate (m3/h), Vf is the volume of the filter bed (m3), and CGi and CGo are the respectively inlet and outlet H2S concentration (ppm; g/m3) (13-17).
In order to investigate the changes in the bacterial population of the packing material, the cultivation of microorganisms were done at the beginning, at the middle, and at the end of the operation system. To cultivate different bacteria, three different media were used as following: (i) the tryptic soy agar (TSA) for the cultivation of heterotrophic bacteria, (ii) thiosulfate oxidizing medium for the cultivation of Thiobacillus thioparus, and (iii) sulfur medium for the cultivation of Thiobacillus thiooxidans. The compositions of the basic media for the cultivation of microorganisms are listed in Table 2.
The thiosulfate oxidizing and sulfur media were prepared according to the pertinent listed in the reference called the Standard Methods for the Examination of Water and Wastewater (18). In order to prepare the thiosulfate oxidizing medium, the pH value was adjusted to 7.8 after sterilization. Meanwhile, sodium thiosulfate (Na2S2O3) and ammonium sulfate [(NH4)2SO4] were separately sterilized and added before use. For the preparation of the sulfur medium, the pH value was adjusted to 4.8 after sterilization. The sterilization was done in an autoclave at 121°C for 20 minutes.
3.3. Determination of Bacterial Population and pH Values
According to statement of Hirai et al. "about 10 g (wet weight) of the samples was collected and homogenized in 90 mL sterilized water at 10000 rpm for 10 minutes". After the preparation of the serial dilutions from 10-10 to 10-1 a mixed and homogenized sample was cultivated in each of the three media through the spreading technique. The media were then put in an incubator at 37°C for 48 hours. Subsequently, the bacterial populations were expressed in colony forming unit per gram (CFU/g) (19).
In this study, the pH values of the filter’s packing materials were measured every five days. Based on the study by Taghipour, 5 g of the packing material from the three sampling ports of each filter was collected. The samples were then mixed with 50 mL of distilled water using a magnetic stirrer for 30 minutes at 350 rpm (20). Then the pH values were measured using a digital pH meter (THERMO, Star Series).
| Properties | Amount |
|---|---|
| Particles size | |
| < 2 mm | 0% |
| 2 - 4 mm | 22% |
| 4 - 6 mm | 24% |
| 6 - 8 mm | 21% |
| 8 - 15 mm | 33% |
| > 15 mm | 0 |
| Density, kg/m3 | 634 |
| pH | 6.8 |
| Analysis of Elements | |
| Carbon | 3.56% |
| Hydrogen | 6.98% |
| Nitrogen | 0.45% |
| Sulfur | 0.043% |
Some Important Properties of the Dried Sewage Sludge Used as a Packing Material
| Media | Amount, g |
|---|---|
| TSA medium (heterotrophic bacteria) | |
| Tryptic soy agar | NAa |
| Thiosulfate Oxidizing Medium (Thiobacillusthioparus) | |
| Na2S2O3.5H2O | 10.0 |
| Dipotassium hydrogen phosphate (K2HPO4) | 2.0 |
| Magnesium sulfate (MgSO4.7H2O) | 0.1 |
| Calcium chloride (CaCl2.2H2O) | 0.1 |
| Ferric chloride (FeCl2.6H2O) | 0.02 |
| Agar | 20.0 |
| Reagent-grade water (1 L) | NAa |
| Sulfur Medium (Thiobacillusthiooxidans) | |
| Sulfur, elemental | 10.0 |
| Potassium dihydrogen phosphate (KH2PO4) | 3.0 |
| Magnesium sulfate (MgSO4.7H2O) | 0.5 |
| Ammonia sulfate (NH4)2SO4 | 0.3 |
| Calcium chloride (CaCl2.2H2O) | 0.25 |
| Ferric chloride (FeCl2.6H2O) | 0.02 |
| Agar | 20.0 |
| Reagent-grade water (1 L) | NAa |
The Compositions of the Basic Media for the Cultivation of Microorganisms
4. Results
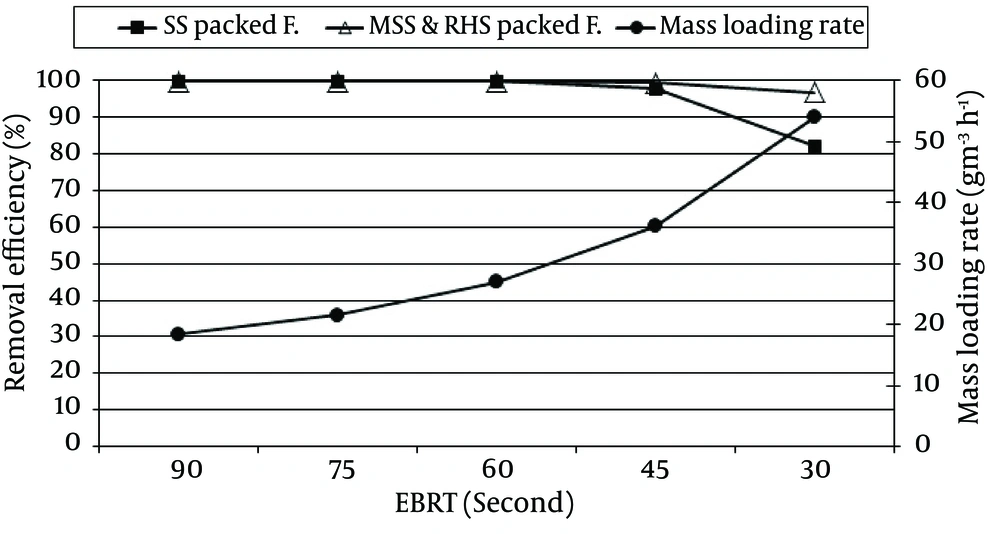
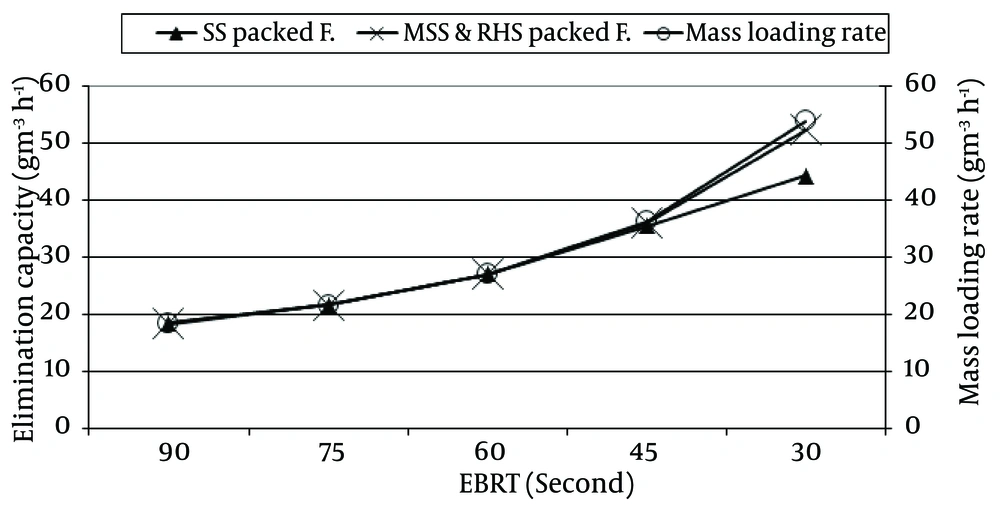
In order to investigate the performance of the two packing materials, namely SS and MSSRHS, the RE and EC of H2S were assessed. The result of RE and EC of H2S versus different EBRTs and different mass loading rate of H2S in the SS and MSSRHS packed filters are shown in Figure 2 and 3, respectively.
In order to investigate the changes in the bacterial population in the packing material, the cultivation of the microorganisms in the three different media (i.e. tryptic soy agar, thiosulfate oxidizing, and sulfur medium) was done at the beginning, at the middle, and at the end of the operation system. The bacterial populations were expressed in CFU/g. The results of the bacterial populations at defined points of the operating time are summarized in Table 3.
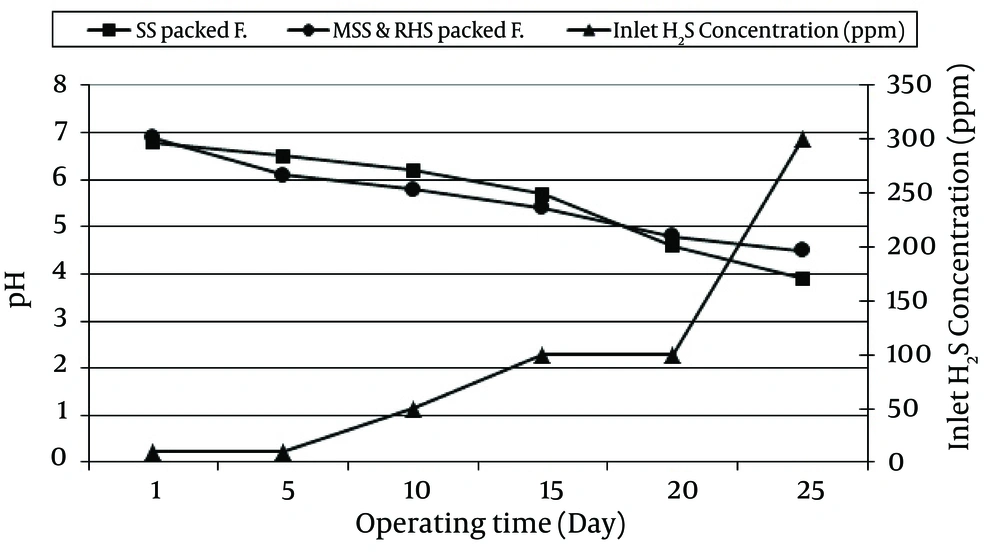
Changes in the pH values versus operating time (day) and different inlet concentrations of H2S in the SS and MSSRHS) packed filters are shown in Figure 4.
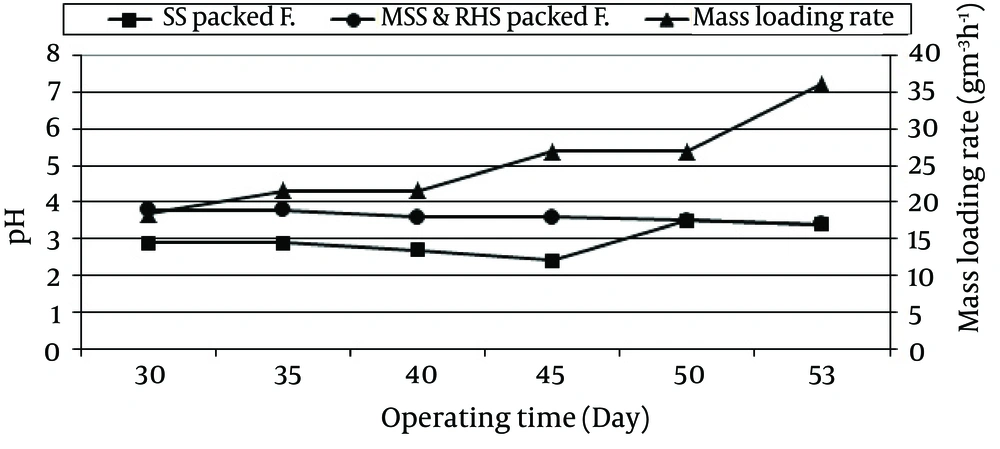
Changes in the pH value versus operating time (day) and different mass loading rates (g/m3h) in the SS and MSSRHS packed filters are shown in Figure 5.
| Media | Sampling Time | ||
|---|---|---|---|
| Beginning | Middle | End | |
| Sewage Sludge Packed Filter | |||
| Tryptic soy agar medium | 8.6 × 109 | 4.6 × 1012 | 9.7 × 105 |
| Thiosulfate oxidizing medium | 4.8 × 103 | 6.1 × 105 | 2.3 × 102 |
| Sulfur medium | 6.2 × 103 | 6.4 × 106 | 6.8 × 103 |
| Mixed Sewage Sludge With Rice Husk Silica Packed Filter | |||
| Tryptic soy agar medium | 8.9 ×107 | 9.5 × 1012 | 8.7 × 109 |
| Thiosulfate oxidizing medium | 2.3 ×102 | 2.6 × 105 | 3.4 × 103 |
| Sulfur medium | 7.6 ×102 | 8.4 × 106 | 8.7× 105 |
A Summary of the Results Gathered for the Bacterial Population in the Biological and Physico-Biologic Filters a
5. Discussion
In this study, after 53 days of operating time and 54 g/m3h mass loading, the pH values in the SS and MSSRHS packed filters were reduced from 6.8 to 1.8 and 6.9 to 3.4, respectively. The reduction of pH values was due to the production of sulfuric acid by sulfur bacteria. Shinabe et al. indicated that sulfuric odorants, such as hydrogen sulfide and methanethiol, are oxidized into sulfuric acid by sulfur bacteria that use these compounds as energy sources (21). Meanwhile, Yang and Allen reported that the "pH at the inlet to their biofilter decreased from 8.0 to 2.5 after 32 days operating time" (22). McNevin and Barford, observed a decrease in the pH value (from 6.5 to 3.5) after 60 days of operating time for their activated sludge biofilter (23). Cho et al. described, deodorizing microorganisms can degrade malodorous gases such as H2S and converted them to the acidic form. Decreasing of pH values into inhabitation level for deodorizing microorganism's activity reduce the deodorization efficiency significantly. Thus, buffering capacity of packing material for biofilter to resist pH changes, are very important in maintaining microorganism activity for long-term biofilter operation (11). In MSSRHS packed filter, sulfur bacteria (both Thiobacillus thioparus and Thiobacillus thiooxidans that have been studied in this study) could grow and oxidize H2S better than SS packed filter could.
In conclusion, the RE and EC of H2S were higher when using MSSRHS as packing material in comparison to SS packing material. This result could be because of lower changes in pH value and higher bacterial population in MSSRHS packing material during the system’s operation.