1. Background
Peripheral nerves sheathed by three layers of connective tissue. The outer layer is the epineurium that covers the nerve, has inter fascicular septum which separates nerve fascicles. Perineurium is the middle layer which covers each nerve fascicle. Perineurium has squamous cell layers; these cells are connected by tight junctions and have basement membranes on both sides. The inner connective tissue layer is the endoneurium that encloses a nerve fiber with its axon and covering Schwann cell (1).Diabetic neuropathy is associated with changes in the extracellular matrix (ECM) and the basement membrane thickening in peripheral nerves (2-4). The ECM provides physical support for cells and tissues and also has a major role in proliferation, differentiation, migration and regulating cell behavior (5). Basement membranes are structures of ECM that cover the basal side of epithelial and endothelial cells. Laminin is one of the major components of all basement membranes. It is a large glycoprotein (~ 800 kDa) distributed ubiquitously in basement membrane and links to other components of the basement membrane. Laminins are heterotrimeric glycoproteins composed of α, β and γ chains. There are currently five α, four β, and three γ chain genes that have been described in vertebrates and the chains can assemble into at least 15 different heterotrimers. Laminins give structure to the basement membrane; provide attachment sites for cells via cell surface proteins e.g. dystroglycan and act as ligands for receptors on cells e.g.integrins, initiating signals that influence cell behavior and survival. Laminin has an essential role in the basement membrane and support regeneration of the nerve (6-8). Long-lived proteins such as laminin are a potential target for glycation (9). The primary cause of diabetic neuropathy is hyperglycemia (3). Hyperglycemia leads to increased oxidative stress, polyol pathway activation, advanced glycation end products (AGE) formation and reactive oxygen species (ROS) production. Polyol pathway converts glucose to fructose via sorbitol production resulting in AGEs formation. AGEs accumulate in both intracellular and extracellular proteins, especially in those with poorly controlled diabetes. Hyperglycemia-induced oxidative stress contributes to the pathology of diabetic neuropathy. ECM proteins of peripheral nerve may alter in the structure and function that induced by hyperglycemia or AGEs. AGEs stimulate production of oxygen free radicals leading to oxidative stress (9-11). Hyperglycemia and increased oxidative stress are resulted in vascular damage and reduced peripheral nerve blood flow (10, 12). Oxygen free radicals activity increased in sciatic nerve in experimental diabetic neuropathy (13). ALA is an antioxidant agent that acts via scavenging of oxygen free radicals (14). Therapeutic approaches using ALA in both prevention and treatment of diabetes and oxidative stress have been reported in human and animal studies (10, 13-15). In experimental models, ALA has been shown to reduce lipid peroxidation, fix deficits in neuropeptides, improve endoneurial blood flow and glucose uptake (13). ALA decreased oxidative stress and improved blood flow in diabetic nerves (14, 15). ALA decreases vascular endothelial growth factor expression and hypoxia in the retina (14). In diabetic nephropathy, ALA has been shown to prevent of renal insufficiency, glomerular mesangial matrix expansion, and glomerulosclerosis (16).
2. Objectives
The aim of the present study was to investigate the laminin alteration in the sciatic nerve of diabetic rats and also the effects of the ALA treatment on improving of blood glucose levels, body weight and laminin expression in sciatic nerve of streptozotocin (STZ) inducing diabetic rats.
3. Materials and Methods
3.1. Animals
24 adult male Wistar rats (200–250 gr body weight, 8-9-weeks-old) were obtained from the animal centre at Mashhad University of Medical Sciences (MUMS). They were maintained under constant conditions with temperature (23-25°C) and 12 hours light-dark cycles with free access to water and food. The study was approved by the MUMS animal ethics committee.
3.2. Experimental Design and Treatment
The rats were randomly divided into three groups as follows: Intact control (C), Diabetic without treatment (D) and Diabetic with ALA treated (D+ ALA) groups. Diabetes was induced in the rats by a single intraperitoneal injection of Streptozotocin (STZ) (Sigma, 55 mg/kg body weight) was dissolved in 0.1 mol/L citrate buffer, pH 4.5. Control rats received citrate buffer alone. The rats were fasting overnight prior to STZ injection. Diabetes was verified by evaluating tail vein blood glucose levels by using a digital glucometer (Accua check, Germany). The rats with a blood glucose level higher than 300 mg/dL were considered as diabetic. The group was treated with ALA (Sigma), the powder was mixed with saline and 100 mg/kg injected intraperitoneally 5 times a week. The treatment of diabetic rats was conducted after the verification of diabetes. Finally all animals were anesthetized and an incision was made in the mid-thigh and sciatic nerves were carefully removed, fixed in 10% neutral buffered formalin solution for 48 hours , embedded in paraffin, sectioned at 5 μm thickness and were mounted on poly-L-lysine slides (Sigma) for immunohistochemical study.
3.3. Immunohistochemical Study
For immunohistochemical study, sections were deparaffinized, rehydrated and rinsed in phosphate-buffered saline solution (PBS) (pH 7.4). Enzymatic antigen retrieval was carried out with Trypsin 0.05% in PBS and preincubated in 0.025% Triton X-100 in PBS for 10 min. To block nonspecific antibody, this was followed by 5% goat serum and bovine serum albumin (BSA) 2% in PBS for 1 hour. Then sections were reacted with primary antibody (Laminin β1 chain antibody, Abcam 11575) diluted 1:250 in PBS with 1% BSA for overnight incubation at 4°C. The next day, sections were washed with 0.025% Triton X-100 in PBS for 10 min. Endogenous peroxidase activity was blocked using 0.03% H2O2 for 15 minutes. Then specimens incubated with goat poly clonal secondary antibody (Abcam 97051) diluted 1:800 in PBS with 1% BSA for 2 hours in room temperature and reacted with 0.03% solution of 3,3-diaminobenzidine tetra hydrochloride (DAB) containing 0.3% H2O2 for 20 minutes. Specimens were washed and then were counter stained with hematoxylin. Finally, sections were washed, air-dried, dehydrated, cleared and mounted in glass slides. Sections stained only with secondary antibody were treated as the negative control. The immunostaining sections photographed by a light microscope (Olympus DP12, Japan) and laminin β1 reaction in the sciatic nerves were evaluated. All nerve samples were graded blind by two observers. Quantitative analysis was performed with grading scores of 0, 1, 2, 3, and 4 representing no, weak, moderate, strong and very strong staining, respectively (17, 18).
3.4. Real Time PCR Study
3.4.1. RNA Extraction and cDNA Synthesis
Sciatic nerve samples were removed and collected to RNA stabilization reagent (RNA later, Qiagen, Germany). Samples were used fresh or kept at –70°C. Total RNA was isolated by the RNeasy Mini Kit (Qiagen, 74104) according to the manufacturer’s instructions. Briefly, 25 to 30 mg of sciatic nerves were lysed with buffer containing 1% mercaptoethanol and homogenized by using polytron homogenizer (PT 1200E, Switzerland) and subsequent ultrasonication. RNA extraction proceeded according to the manufacturer’s instructions. The RNA integrity was checked by visualization of 18S and 28S ribosomal bands on 1% agarose gel. First strand cDNA were made by using a cDNA synthesis kit (Fermentas) according to the manufacturer instructions.7 μL of total RNA with 1 μL of random primer and 3 μL of water were mixed and incubated at 65°C for 5 min and then add the filling component: 4 μL of 5x reaction buffer,1 μL of ribolock RNase, 2 μL of 10mm dNTP mix,1 μL of reverse transcriptase and then incubated for 15 minutes at 25°C followed by 60 min at 42°C and terminated reaction by heating at 70°C for 5 minutes. cDNA samples were stored at -20°C.
3.5. Real-time Polymerase Chain Reaction (PCR)
Real-time PCR was performed by using the Stratagene Max3000p (USA). The reaction mixture (total volume of 20 μL per well) consisted of :10 μL of SYBR Green PCR Master Mix (Pars Tous, Iran), 1 μL of each forward and reverse primer, 0.25 μL of Taq DNA polymerase , 6.75 μL of H2O and 1μL of cDNA template. The following primers were used:
Laminin β1, 5'-AAGGAGGCGTTGGAAGAAGCAG-3' (forward) 5'-GGGAGGCATTGGTCAGGGTTC-3' (reverse)
GAPDH, 5'-AACTCCCATTCTTCCACCTTTG-3' (forward) 5'-CTGTAGCCATATTCATTGTCATACCAG-3' (reverse)
The PCR amplification conditions were as follows: pre-denaturing at 94°C for 10 minutes followed by 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds and 72°C for 45 seconds. At the end of the runs, the temperature was 95°C to construct a melting curve. The relative amount of each mRNA was normalized to the house keeping gene, GAPDH. The average of the relative amount of each mRNA in control group is defined as 1.0.
3.6. Statistical Analysis
The data were analyzed by using SPSS software. All values are expressed as mean ± SEM. Statistical evaluation was carried out by using one way ANOVA followed by Tukey test. P values < 0.05 was considered significant.
4. Results
4.1. General Characteristic of Animals
Our study indicated that the blood glucose levels were increased significantly and body weights reduced markedly in untreated diabetic rats compared to control rats (P<0.001) (Table 1). While the ALA treatment in D+ ALA group decreased blood glucose levels significantly compared to untreated diabetic group (P<0.05), it has no significant effect on the weight gain in diabetic rats (Table 1).
4.2. Results of Immunohistochemical Study
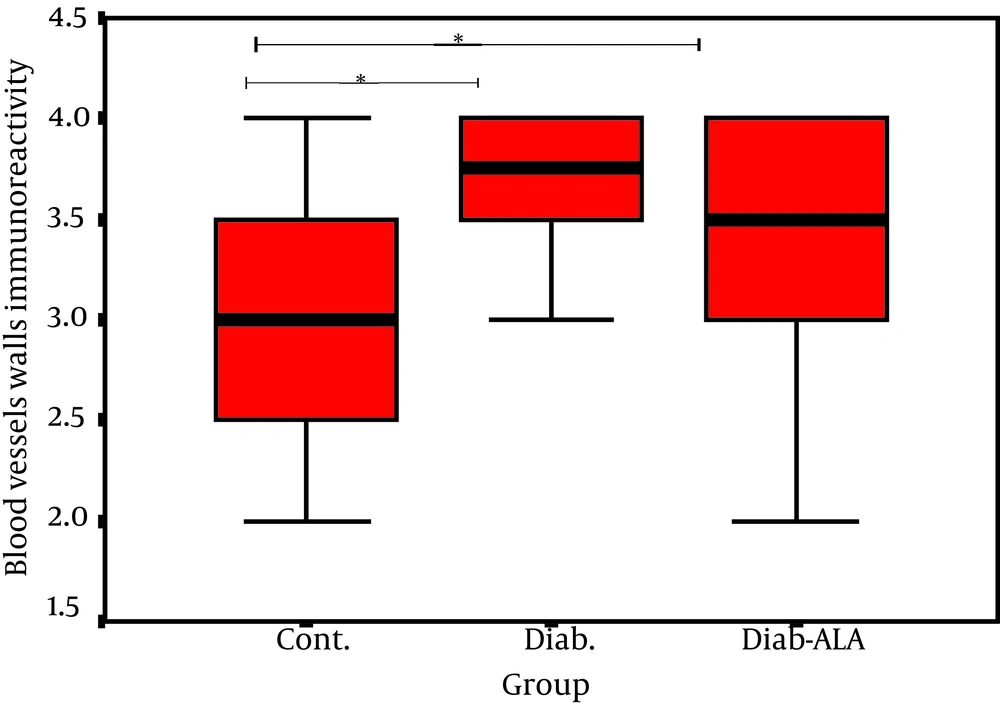
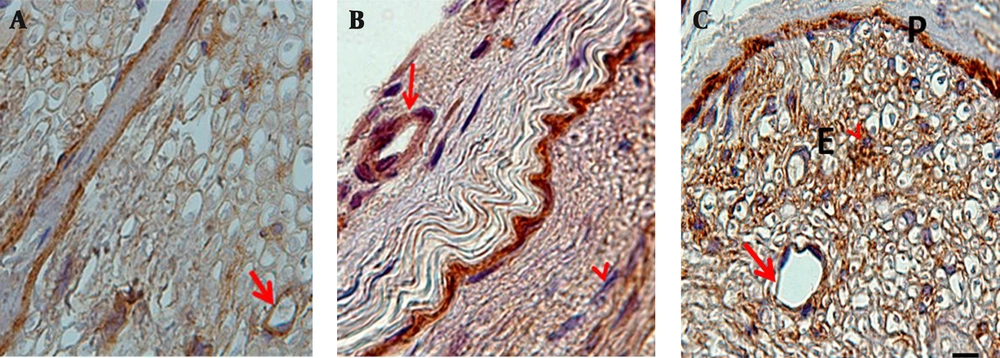
In this study we investigated the localization of laminin in the fascicles with similar sized sciatic nerves, according to the intensity of color darkness. Our findings revealed that reaction for laminin in normal sciatic nerves was present in the perineurim, around the Schwann cells and in the walls of epineurial and endoneurial blood vessels. In diabetic sciatic nerves, laminin was prominent especially in the inner layers of the perineurium and thickened layers of basement membranes of the epineurial andendoneurial blood vessels (Figure 2). ALA treatment decreased significantly laminin expression only in the walls of epineurial and endoneurial blood vessels (P < 0.05) (Figure 1). In normal sciatic nerve, fibroblasts were seen in the epineurium and endoneurium and they were different in shape and size. In untreated diabetic rats and in D+ALA group, fibroblasts were increased in number particularly around the epineurial and endoneurial blood vessels. Moreover, a stronger reaction was seen in association with endoneurial fibroblasts (Figure 2).
4.3. Results of Real-Time PCR Study
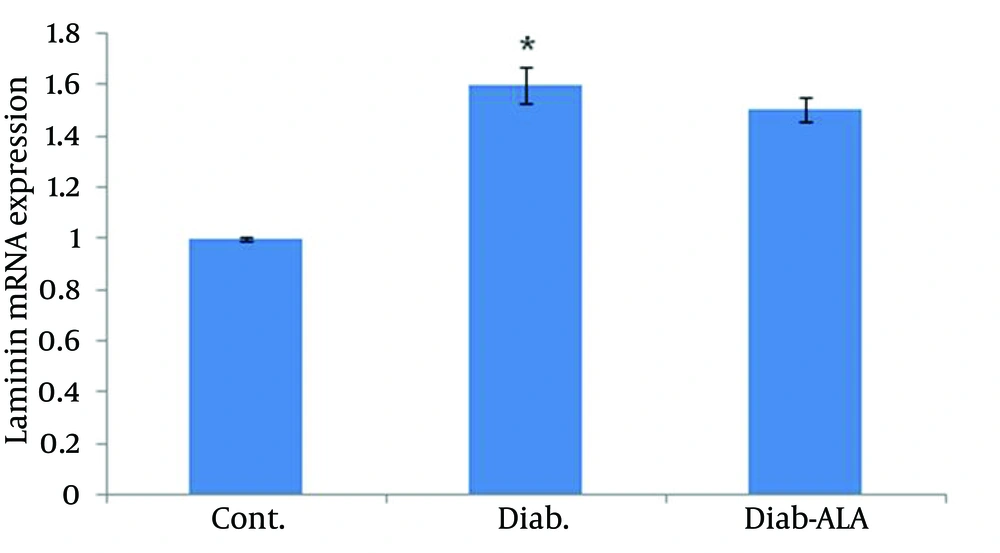
laminin β1 mRNA expression in sciatic nerves were detected by real-time PCR. Data analysis revealed that a 0.6 fold increasing in laminin β1 mRNA level in the untreated diabetic group compared to the control group. Although ALA treatment reduced laminin β1 mRNA over expression, the results were not significant (Figure 3).
5. Discussion
The present study was designed to investigate the therapeutic effects of ALA treatment on blood glucose levels, body weight and alteration in laminin expression of sciatic nerve in the STZ-induced diabetic rats. Unsuccessful nerve regeneration in diabetic neuropathy suggested that it may be in part due to changes in extracellular matrix (ECM) composition (8). ECM proteins of peripheral nerve may cause alterations in the structure and function that is induced by hyperglycemia or advanced glycation end products (AGEs) (9). AGEs stimulate production of oxygen free radicals, leading to oxidative stress. Alterations in the sorbitol, Polyol pathway activation, decreased nitric oxide and impaired (Na+/K+) ATPase activities are some of other pathogenetic mechanisms that have been implicated in diabetic neuropathy (14, 19). ALA delays or reverses diabetic neuropathy through its multiple antioxidant properties (11). Our findings revealed that ALA with 100 mg/kg, intraperitoneally injection has shown partial hypoglycemic effects in the diabetic rats. This may be due to increase glucose transport by ALA (20). Sugimoto et al. showed that rat sciatic nerves had rich laminin content (21). Glycation of laminin in ECM leads to impaired regenerative activity in diabetic neuropathy (21). Previous studies have suggested that the level of laminin mRNA increased in kidney or retina after diabetes induction (4, 22-25). Our immunohistochemial results indicated that diabetes in rats resulted in significantly increased laminin expression in the perineurium, endoneurium, epineurial and endoneurial blood vessels. Some researchers have reported diabetic neuropathy resulted in abnormal deposits of laminin in human and animal models (8), but in contrast Hill et al. reported that, in human diabetic neuropathy laminin content in diabetic and control nerves was not significantly different (6). Also Serafin et al. (26) assessed laminin in peripheral nerves of STZ-induced diabetic mice, and reported that it did not differ in laminin expression in the diabetic and control nerves. These discrepancies are probably due to differences in experimental methods, in Serafin et al. study (26), mice was made diabetic with a low dose of streptozotocin with a short study time period and in Hill et al. study (6), duration of diabetes in diabetic patients was different. Diabetes duration and long-term hyperglycemia are the most important reasons for polyneuropathy (22). Beneficial effect of ALA on the neuropathic symptoms due to diabetic neuropathy had been reported (10, 11, 13). Our immunohistochemical results showed that ALA treatment decreased significantly laminin expression only in the basal membrane of epineurial and endoneurial blood vessels. This may be due to the fact that the antioxidants reduce vascular impairment in diabetic rats (23). Oxidative stress has been suggested to contribute to defective nerve blood supply and ALA has been shown to protect peripheral nerves from ischemia in experimental diabetic neuropathy (10, 11). In agreement to our results, , some researchers showed that ALA prevents the vascular complications in the STZ induced diabetic rats (10, 13, 15). Previous studies have suggested that the level of laminin β1 mRNA in kidney or retina increased after diabetes induction (4, 24, 25). Also our findings showed that, 12 weeks after diabetes induction, laminin β1 was increased in sciatic nerve of untreated diabetic rats at mRNA level. Also ALA treatment only partially decreased laminin β1 over expression at mRNA level in sciatic nerves. Diabetic neuropathy is a multi-factorial disorder and our findings suggested that ALA with 100 mg/kg dose had no significant effect on improvement of laminin β1 mRNA regulations in diabetic nerves. This may also be due to inadequate amount or duration of ALA treatment.This experiment showed that laminin glycoprotein is expressed in rat sciatic nerves, and the expression of laminin is increased in sciatic nerves of STZ-induced diabetic rats. It might be suggested that ALA treatment reducing the laminin up-regulation only in the epineurial and endoneurial blood vessels walls. Further investigation in this way is warranted.