1. Background
Language is a complex process involving a number of processing steps. While chronometric behavioral experiments allow to investigate the end point of the time-course of cognitive processes of language, the continuous measure of brain activity in Event-related Brain Potentials (ERP) studies allows direct and temporally precise insight into the cognitive processes (1).
The ability to name an object is a basic language skill (2). Based on the model by Levelt and Indefrey picture naming starts by activating some lexical concept and selecting it for expression, which takes 175 milliseconds; the next stage (called Lemma) involves accessing the syntax of the target word, where a word associated with the lexical concept of selected, that took 75 ms. After choosing the Lemma, phonological encoding starts in the next 80 ms, the sound features associated with the target word. Finally, the syllabic information and prosody are accessed, which takes about 125 ms, and then there is phonetic encoding that lasts 145 ms and ends with production of the word (3). Therefore, it takes about 600 ms, but this time depends on many factors such as word frequency and the word Age of Acquisition (AoA) (4). Some studies reported that Early Acquired Words (EAW) were named faster than Late Acquired Words (LAW), and the words acquired earlier in life are more reversible after a brain injury (5, 6). In many tasks the effects of frequency and AoA on reaction latencies are equal, but in picture naming the effect of AoA is greater than frequency (7). As far as the locus of AoA effects in picture naming is concerned, the phonological retrieval stage is the one proposed most frequently. Previous studies reported no reliable AoA effects in the tasks indexing the pre-lexical and post-lexical levels involved in picture naming, most likely locus of AoA effects must be lexical (8). Morrison and Ellis tested a post-lexical (articulatory) locus of AoA effects at delayed word naming task and since no reliable AoA effect was found, an articulatory locus of AoA effects was excluded (9). Belke studied the effects of semantic context on early and late-acquired words in two tasks of naming pictures and naming words. He reported that the effect of AoA was greater in naming pictures than words, and the effect of semantic context in naming early-acquired words was more substantial than in late-acquired ones. This effect was not found in word reading, since word reading does not involve Lemma stage (7). In addition, Morrison investigated the hypothesis of the AoA effect semantic locus by picture categorization task and reported no effect of AoA (10). Since the role of acquisition order in the activation speed of semantic representation was not supported in picture naming, semantic locus of AoA effects was rejected (7).
According to the assumption that lexical access involves Lemma selection and lexeme retrieval, AoA effects can take place either at the Lemma and phonological levels or the links relating the two levels. Gerhand et al. favored a phonological locus of AoA effects given the assumption that Lemma access is not required in word reading, whereas both word reading and picture naming obligatorily require phonological retrieval (11). Furthermore, Chalard and Bonin investigated the semantic locus of AoA effect, but the results did not support the semantic locus of AoA effect (8). In accordance with the phonological locus of AoA effect, Brown and Watson developed the phonological completeness hypothesis. According to this hypothesis, the Early-acquired Words (EAW) are holistic in nature, whereas the LAW are stored differently in a segmented manner (12). The phonological completeness account suggests that LAW should be segmented faster than EAW. However, Mobaghan and Ellis found evidence contrary to this view, using a phonological segmentation task in adults (13). Johnston and Barry also suggested it is not possible to attribute the AoA effect exclusively to the retrieval mechanism. They also reported that semantic categorization only affects the speed of categorization of pictures but has no effect on the speed of naming pictures (14). However, Laganaro reported that AoA influences immediate naming and this impact emerges within the phonological encoding time window, but observed no AoA effect on delayed naming (1).
Most of the ERP studies that investigate speech production do not use the method of overt and immediate production of pictures due to the artifacts created in ERP signals during motor or execution preparation. They, instead, tend to apply metalinguistic tasks and silent or delayed production techniques (1). In either case, it is possible to prevent artifact generation, but the time range of the processes changes compared with those of the tasks done by the overt production (2). In addition, terminal processes (phonetic and phonological encoding) may not be done completely (4). Only a few studies used overt production (immediate picture naming) (1, 15-18). Therefore, to investigate phonological process of naming an overt production should be used to capture the terminal process of naming.
2. Objectives
Since it was hypothesized that the locus of AoA effect is phonological encoding, the current study aimed to thoroughly investigate amplitude, latency and the scalp distribution of ERP waves during overt pictures naming to determine the time periods where amplitude differences were found between AoA conditions. The investigation of brain event-related potentials in naming pictures can provide primary data and compare the preliminary ERPs data with potentials of individuals with naming disorders and find the time period where differences were found and therapeutic strategies were presented based on that.
3. Patients and Methods
3.1. Subjects
The subjects were 15 males, native Persian speakers, aged 18 - 25 years. All were right-handed as determined by Edinburgh handedness scales (18). Based on the questionnaires, interviews, and examinations, subjects did not have any neurological or motor problems, history of psychotropic substance use or head injury. Since in case of long hair, gel injection and decreasing impedance is more difficult and may cause harm to the results of the research, only males were enrolled in this study. All subjects signed the informed consent form.
3.2. Stimuli
Stimuli of the study were 80 pictures selected from the standard set of the research by Tahanzadeh et al. (19). Half of the items were EAW (mean < 4 years old) and the other half LAW (mean ≥ 4 years old). Early and late-acquired words were matched regarding word frequency, name agreement, semantic relations and length in phonemes.
3.3. Procedure
The participants were tested individually in a sound proof dark room without any extra stimuli. They kept 60 cm far from the screen. Black and white pictures in constant size of 9.5 × 9.5 cm were presented on a gray screen in a random sequence. A grey screen was used to avoid extreme light exposure.
Before the experiment, subjects were familiarized with the experimental pictures and their corresponding names as a demo. The experimental trial had the following structure: the protocol of presentation was: first a + sign was shown for 1000 ms to prepare for the test and prevent excessive eye movements. Then the picture appeared on the screen and remained for 1000 ms. The participant had to produce the word corresponding to the picture immediately once he saw it. Next, a gray screen appeared on screen lasting for 2000 to 3000 ms (randomly) to create a gap between pictures.
After putting the head cap to record the waves, two reference electrodes were placed on the mastoid bone and then TEN20 gel was placed behind the mastoid electrodes. In order to reduce contact impedance, NUPREP gel was injected into the head cap electrodes. Finally DCI gel was injected into those electrodes, and impedances were kept below 5 kilo ohm. During the test, the subjects were required to minimize their additional body movements.
The given responses were recorded using the voice key to investigate the voice Reaction Time (RT). Simultaneously, EEG (electroencephalogram) signals were recorded. For every subject, the responses related to each of the 80 pictures were checked individually and after elimination of errors, RT (the time distance between the onset of the picture and the onset of name production) was obtained.
EEG was recorded continuously from 64 electrodes mounted on an electrode cap according to the international 10 - 10 system using the “ERP BE PLUS 64” device (EBNeuro Company, Italy) (Figure 1). Signals were sampled at 250 Hz (samples per second) with band-pass filters set between 0.16 and 100 Hz.
In addition to automatically rejecting the epochs with amplitudes reaching ± 100 microvolt, the wrong answers including producing wrong names, verbal non-fluency, and failure to record the response were excluded from averaging and epochs with a high noise level or large electrode drifts detected from visual inspection were excluded from further analysis.
Finally, every subject had approximately 35 trials for each of the LAW and EAW. Evoked potentials corresponding to pictures related to each group were averaged using the Galileo Software (Galileo company, Italy). Then epochs of 300 ms with respect to RT of each trial were selected for each subject and AoA condition. This time window is related to the phonological encoding and syllabification stage (after Lemma retrieval and prior to phonetic encoding) and started at 400 ms before the production onset of each individual trial and ended at 100 ms before the production onset. Since the analyzed period stopped before the subjects started to articulate, possible artifacts during motor execution were avoided. Replicable waves were identified in each epoch of the aforementioned 300 ms time window. These waves were clearly different from the background waves and had a certain spatiotemporal patterns in all of the electrodes. Amplitude, latency, and scalp distribution of these time points were investigated. Based on the Regions of Interest (ROI), the electrodes were divided to four groups: Left Anterior (LA), Right Anterior (RA), Left Posterior (LP), and Right Posterior (RP) (Table 1). The ERPs were first subject to determine the time periods where amplitude differences were found between AoA conditions, and second, whether the scalp distribution is different in various regions of interests or not. Repeated Measurement ANNOVA and paired t-test were used to answer these questions.
4. Results
The current study recorded the response time by the voice key for each of the 15 subjects and for all of the 80 pictures. The average reaction time for EAW and LAW were 739.73 ± 73.34 ms, and 779.88 ± 98.52 ms, respectively. Therefore, based on the result of the hypothesis testing with t-test, the effect of AoA was significant (P = 0.022) (Table 2). By investigating the amplitude, latency, and scalp distribution of replicable waves, it was observed that the waves related to early and late-acquired words were different in three time periods, indicated as A, B, C in this paper.
| Regions of Interest | Electrodes |
|---|---|
| Right anterior | AF4, AF8, F2, F4, F6, F8, FC2, FC4, FC6, FC8, C2, FT8 |
| Left anterior | FP1, AF3, AF7, F1, F3, F5, F7, FC1, FC3, FC5, FC7, C1, FT7 |
| Right posterior | C4, C6, T4, CP2, CP4, CP6, TP8, P2, P4, P6, T6, PO4, PO8, O2 |
| Left posterior | T3, C3, C5, CP1, CP3, CP5, TP7, P1, P3, P5, T5, PO3, PO7, O1 |
| Type of Pictures | The Average Response Time | Standard Deviation | P Value |
|---|---|---|---|
| Early acquired | 739.73 | 73.34 | 0.022 |
| Late acquired | 779.88 | 98.52 |
Different latency between early and late acquired words appeared in three time spans: 1) Point A is related to the time approximately 400 ms after stimulus presentation (about 300 ms prior to production); 2) Point B is related to the time approximately 500 - 550 ms after stimulus onset. According to Indefrey and Levelt the differences in latency observed in A and B fall within the time-window estimated for phonological encoding process (20); 3) Point C is related to the time approximately 600 - 650 ms after stimulus presentation.
In pictures related to early-acquired words, wave A appeared 41 ms (P = 0.009) in the left posterior, and 48 ms (P = 0.001) in the right posterior sooner than in the pictures related to late-acquired words (Table 3). Furthermore, in pictures related to early-acquired words, wave B appeared 46 ms (P = 0.012) in the left posterior and 44 ms (P = 0.003) in the right posterior sooner than in the pictures related to late-acquired words. In pictures related to early-acquired words, wave C appeared 44 ms in the right posterior sooner than in the pictures related to late-acquired words (P = 0.027) (Table 3).
| Type of Pictures | Mean | Standard Deviation | Signal |
|---|---|---|---|
| Pair 1 | 0.165 | ||
| EALA | 633.39 | 91.83 | |
| LALA | 682.17 | 111.76 | |
| Pair 2 | 0.068 | ||
| EARA | 618.33 | 91.026 | |
| LARA | 665.43 | 100.71 | |
| Pair 3 | 0.009 | ||
| EALP | 584.85 | 94.84 | |
| LALP | 625.83 | 102.91 | |
| Pair 4 | 0.001 | ||
| EARP | 565.84 | 107.56 | |
| LARP | 613.79 | 109.1 | |
| Pair 5 | 0.097 | ||
| EBLA | 543.44 | 117.53 | |
| LBLA | 583.50 | 126.93 | |
| Pair 6 | 0.330 | ||
| EBRA | 567.77 | 111.8 | |
| LBRA | 597.80 | 128.68 | |
| Pair 7 | 0.012 | ||
| EBLP | 506.32 | 101.61 | |
| LBLP | 562.46 | 108.82 | |
| Pair 8 | 0.030 | ||
| EBRP | 500.66 | 106.97 | |
| LBRP | 544.29 | 132.57 | |
| Pair 9 | 0.773 | ||
| ECLA | 538.23 | 76.86 | |
| LCLA | 545 | 105.02 | |
| Pair 10 | 0.394 | ||
| ECRA | 497.75 | 114.81 | |
| LCRA | 533.75 | 111.83 | |
| Pair 11 | 0.291 | ||
| ECLP | 436.82 | 118.23 | |
| LCLP | 454.20 | 122.97 | |
| Pair 12 | 0.027 | ||
| ECRP | 500.25 | 126.427 | |
| LCRP | 544.08 | 139.717 |
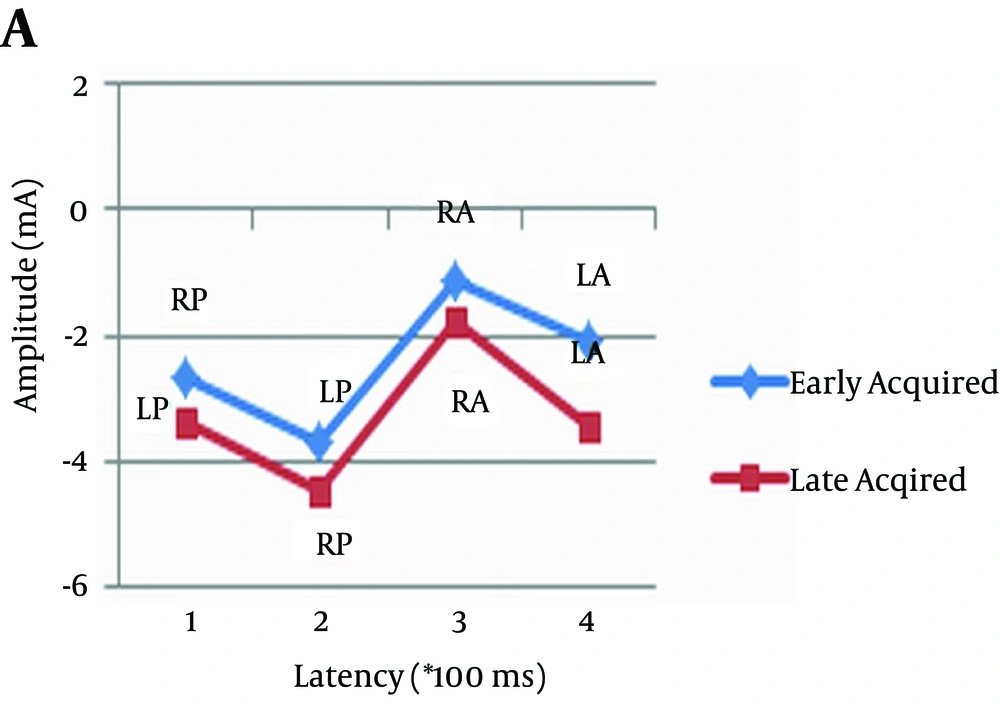
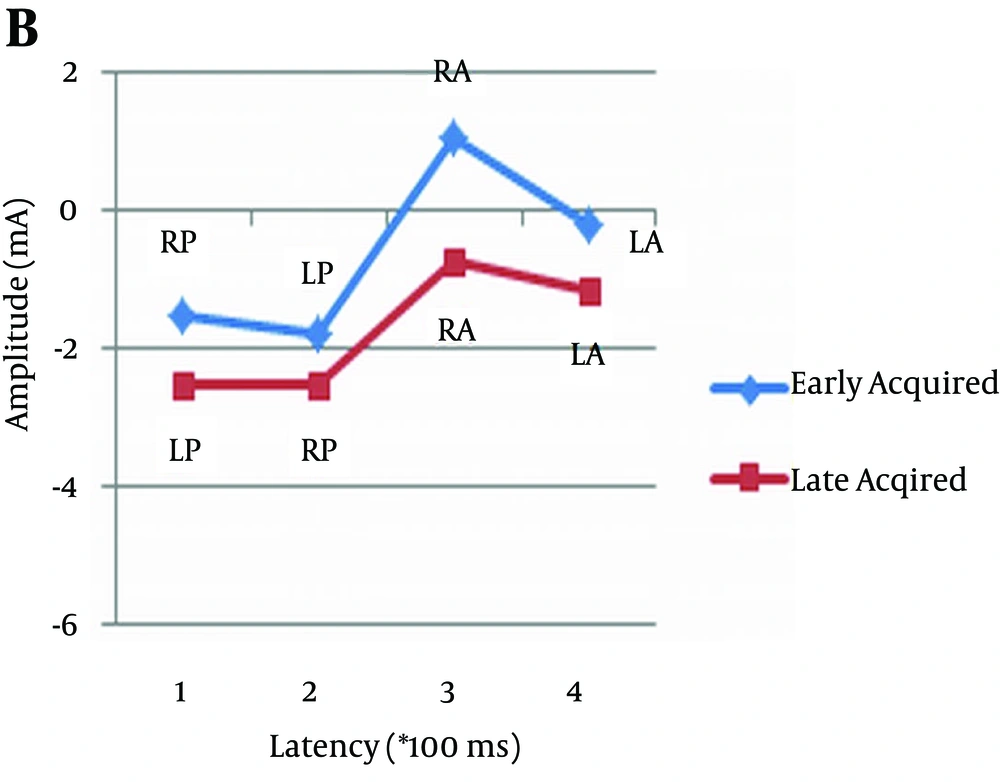
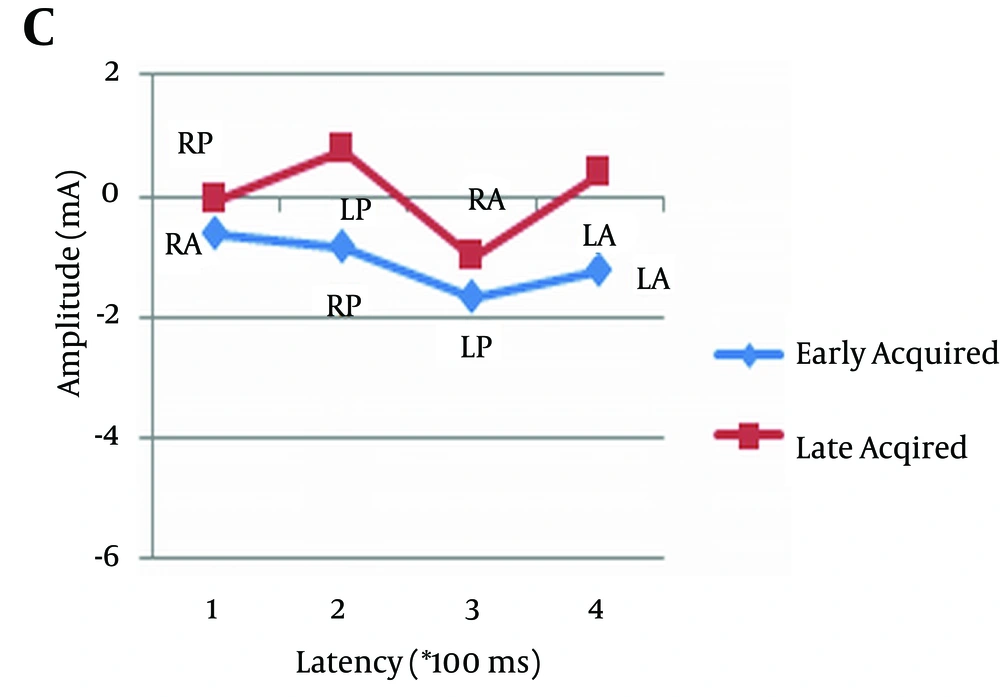
As can be observed in Figures 2, 3, and 4, and Table 4 amplitude difference between early and late acquired words was not statistically significant. In other words, there was no significant difference between the two groups of pictures in terms of the amplitude of event-related potentials.
| Type of Pictures | Mean | Standard Deviation | Signal |
|---|---|---|---|
| Pair 1 | 0.062 | ||
| EALA | -1.36 | 2.08 | |
| LALA | -0.45 | 2.38 | |
| Pair 2 | 0.820 | ||
| EALP | -3.54 | 5.48 | |
| LALP | -3.79 | 4.57 | |
| Pair 3 | 0.882 | ||
| EARA | -0.61 | 1.56 | |
| LARA | -0.50 | 1.72 | |
| Pair 4 | 0.769 | ||
| EARP | -2.66 | 5.38 | |
| LARP | -2.93 | 4.78 | |
| Pair 5 | 0.404 | ||
| EBLA | -0.53 | 1.34 | |
| LBLA | -0.76 | 1.89 | |
| Pair 6 | 0.580 | ||
| EBLP | -1.81 | 5.21 | |
| LBLP | -2.32 | 4.1 | |
| Pair 7 | 0.291 | ||
| EBRA | 0.33 | 2.22 | |
| LBRA | -0.66 | 1.86 | |
| Pair 8 | 0.272 | ||
| EBRP | -1.08 | 4.75 | |
| LBRP | -2.1 | 3.429 | |
| Pair 9 | 0.884 | ||
| ECLA | 0.74 | 1.64 | |
| LCLA | 0.64 | 1.04 | |
| Pair 10 | 0.401 | ||
| ECLP | -1.75 | 4.42 | |
| LCLP | -0.71 | 2.55 | |
| Pair 11 | 0.070 | ||
| ECRA | 0.53 | 1.95 | |
| LCRA | -0.91 | 1.46 | |
| Pair 12 | 0.558 | ||
| ECRP | -0.83 | 4.89 | |
| LCRP | -0.27 | 3.16 |
The amplitude in different brain areas in A, B, and C waves was investigated regardless of AoA. The amplitude of wave A was different in various brain regions (P = 0.002) and the highest, and lowest activities were observed in the left posterior the right anterior (P = 0.002) regions, respectively. In wave B the highest, and lowest activities were also observed in the left posterior and the right anterior (P = 0.032) regions, respectively. However, in wave C the difference between the amplitude of the four regions was not statistically significant (P = 0.69).
5. Discussion
The current study investigated the time-course of AoA effects during picture naming by investigating ERPs at phonological time window in overt picture naming. The results indicated that the early-acquired words were named 40 ms faster than late-acquired ones. Belke studied the response time to early and late-acquired pictures, which included two groups of homogenous pictures (from one semantic category like lion and cat) and heterogeneous (from different semantic categories) (7). Since in the current study the pictures belonged to different classes, it is possible to compare the results of the study with those of the heterogeneous pictures group, where the heterogeneous early-acquired pictures were named 60 ms faster than late-acquired ones. In a study by Chalard and Bonin , the early-acquired pictures were named 87 ms sooner than late-acquired ones (8). Johnston and Barry also investigated the early- and late-acquired pictures in two semantic categories of inside and outside home. In their study, the early-acquired pictures within the inside home group were named 57 ms faster, and within the outside home group were named 105 ms faster than late-acquired pictures (14). Laganaro reported that the time difference between naming early- and late-acquired words was 26 ms (1). This difference between the results of various studies can be due to other variables of visual tasks such as the visual complexity of the pictures, the number of phonological proximities, and non-lexical variables such as the length of phonemes.
Morrison studied AoA effect and word frequency, and reported that AoA is the main determiner of naming rate. However, in the task to classify items into natural and man-made classes, the semantic category was the only variable that had a significant effect on the reaction time (9). Bonin studied the influence of nine variables on naming latency and concluded that the main determiners of the latency of picture naming were the variety of pictures and AoA (21). In a study by Cuetos it was observed that the late-acquired words produce more negative amplitude than the early-acquired ones, as late as 400 to 610 ms after stimulus onset (22).
In the overt picture naming, the AoA modulated response time, latency, and the ERP signal amplitudes. As explained in the ‘‘introduction section, two possible loci of AoA effects in picture naming are suggested: lexical-semantic (Belke et al. (7) and Johnston and Barry (14)) and lexical phonological encoding processes (Chalard and Bonin; Morrison and Ellis, and Morrison et al. (8-10)). The difference between the amplitudes of early and late acquired words was observed in A, B, and C points, but the difference was not statistically significant; point A (about 400 ms after the stimulus onset), point B (about 500 - 550 ms after the stimulus onset), and point C (about 600 - 650 ms after the stimulus onset). The amplitude difference in the time period of A and B was related to the phonological-lexical process, but point C does not apply to any of the time periods related to the processes assumed to be affected by AoA. The information obtained from the latency of A, Band C emergence also indicates earlier appearance of A and B waves.
Therefore, the combination of the information regarding latency and average was in line with the hypothesis of phonological-lexical locus of the AoA effect, since both of them indicate the difference between early- and late-acquired pictures in the time period related to phonological-lexical process during picture naming. The emergence latency of A and B waves was an average of 40 ms shorter in late-acquired words, indicating that the phonological-lexical encoding was longer for late-acquired words than early-acquired ones. These results can be directly linked to the 40 ms difference observed in behavioral results, as the difference in latency between AoA conditions is very close to the difference observed in Reaction Time. In general, the AoA changes the response time (the early-acquired words were named sooner than the late-acquired ones), implying the prolonged process time of late-acquired words that can be attributed to the longer latency observed in the time period of phonological encoding.
According to the investigations, only three studies have explored AoA effects with ERPs, one with a lexical decision task (23), the other with a word reading task (22) and the last with overt naming task (laganaro 2011) (1). Cuetos suggested the phonological-lexical locus for the effect of AoA, but the results of his study were not comparable with those of the present study because of the difference in task type. On the other hand, it is possible to compare the results of the current study with those the ones that investigated the effect of word frequency using ERP, such as Strijkers. Since both variables affect the picture naming time and are highly correlated (18), Strijkers analyzed the effect of word frequency in bilingual speakers during overt picture naming and reported the immediate effect of word frequency within the time range of 180 to 200 ms, which is related to the range of phonological-semantic processes (17). It seems that lexical frequency affects lexical selection, while AoA does not have a significant effect on phonological encoding.
In summary, it can be stated that the AoA changes the response time to naming picture. In other words, the words acquired earlier in the developmental stages were named sooner and phonological encoding was done faster in them and the current study supported the phonological-lexical locus of the AoA effect.