1. Introduction
Glycine encephalopathy, also known as non-ketotic hyperglycinemia (NKH) represents a disorder characterized by elevated concentrations of glycine in all body tissues, especially in plasma and cerebrospinal fluids (1-3). NKH is caused by deficiency in the glycine cleavage system (GCS). Most glycine encephalopathy cases occur during the neonatal period (3, 4). The neonatal form manifests in the first hours to days of life with progressive lethargy, hypotonia, myoclonic jerks, hiccups and apnea, which often lead to coma or death (5). Though NKH is a well-documented entity, to the best of the authors’ knowledge, mortality occurs up to 50 % during the first week of life (3). Surviving infants have profound intellectual disability and intractable seizures (6). Reliable and accurate diagnosis depends on careful interpretation of laboratory findings. The clinical suspicion should lead to determination of glycine in plasma and cerebrospinal fluid (7). Amino acid analysis presents diagnostic values for classic non-ketotic hyperglycinemia, but it should also be performed in suspected cases of atypical non-ketotic hyperglycinemia and in children with seizures, failure to thrive, behavior problems, and uncoordinated movements (7, 8). The current report presents a case of NKH neonatal intractable seizures. Evaluating a sick neonate who presents with hypotonia, encephalopathy, and seizure is a diagnostic challenge; a high index of suspicion for timely diagnosis and treatment could prevent severe complications.
2. Case Presentation
The patient was a four-day-old baby girl in Iran admitted to the hospital due to hypotonia, lethargy and seizures. The patient was born through a cesarean, with premature rupture of membranes (PROM) more than 18 hours, gestational age of 38 weeks, 3150 g weight, 50 cm height, head circumference of 35 cm by 9.10 Apgar and discharged from hospital in good condition and after breastfeeding. She was the baby of a healthy father and the result of a first pregnancy. Parents of the patient were relatives, with no history of inherited diseases. The mother had no problems and illnesses during pregnancy and due to the onset of labor and PROM and lack of progress underwent cesarean. On the third day after discharge, the baby gradually lost the ability to suck and got sleepy and had weak cry, and eventually suffered from organ jump and seizures. She was hospitalized and placed under investigation for sepsis on admission, during the hospitalization the patient was very drowsy and was not responding even to painful stimulus.
Repeated leaping moves were visible in the limbs and there was no reflexes, pupil was average size, and had a poor response to light and deep muscle reflexes were poor. Patient stayed nil per os (NPO), sepsis tests were sent to her and antibiotic therapy (ampicillin and cefotaxime) was started to control phenytoin seizures. Respiratory acidosis was observed in the experiments, but other tests were normal (Table 1). Transfontanelle sonography was reported as normal and therefore brain computed tomography (CT) scan was performed and nothing was observed other than white edema matter. Maternal and fetal TORCH (Toxoplasmosis, Other agents (syphilis, varicella-zoster, parvovirus B19), rubella, cytomegalovirus, and herpes simplex) tests are reported as normal, then metabolic blood-urine tests and magnetic resonance imaging (MRI) were performed.
| Test | Result | Unit | Reference |
|---|---|---|---|
| Lactate dehydrogenase | 798 | IU/L | 250 - 500 |
| Creatine phosphokinase | 189 | IU/L | 24 - 170 |
| Aldolase | 10.6 | IU/L | 2 - 8 |
| Plasma lactate | 17.1 | mg/dL | 4.5 - 20 |
| Blood amonia | 98 | mcg/dL | 79 - 110 |
| HPLC assay for plasma | |||
| Aspartic acid | 44.6 | µM/L | 0 - 40 |
| Glutamic acid | 145.9 | µM/L | 10 - 190 |
| Aspargin | 32.9 | µM/L | 24 - 60 |
| Serine | 221.6 | µM/L | 90 - 250 |
| Glutamine | 203.1 | µM/L | 410 - 960 |
| Histidine | 77.1 | µM/L | 40 - 120 |
| Glycine | 809.2 | µM/L | 220 - 520 |
| Arginine | 53.6 | µM/L | 20 - 160 |
| Alanine | 377.7 | µM/L | 200 - 600 |
| Tyrosine | 50.8 | µM/L | 30 - 140 |
| Alfa amino butiric acid | 18.8 | µM/L | 8 - 37 |
| Valine | 148.4 | µM/L | 110 - 300 |
| Phenylalanine | 87.7 | µM/L | 30 - 100 |
| Isoleucine | 49.7 | µM/L | 20 - 130 |
| Leucine | 127.7 | µM/L | 40 - 230 |
| Ornithine | 79.7 | µM/L | 20 - 135 |
| Lysine | 180.2 | µM/L | 60 - 250 |
| Citrulline | 22.4 | µM/L | 8 - 36 |
| Threonine | 175.0 | µM/L | 46 - 222 |
| CSF glycine | 138.8 | mM/L | 3.7 - 7.6 |
Abbreviations: CSF, cerebrospinal fluid; HPLC, high-performance liquid chromatography.
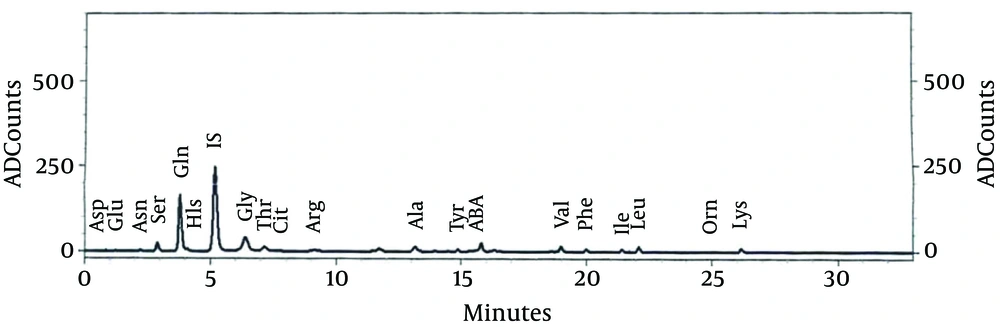
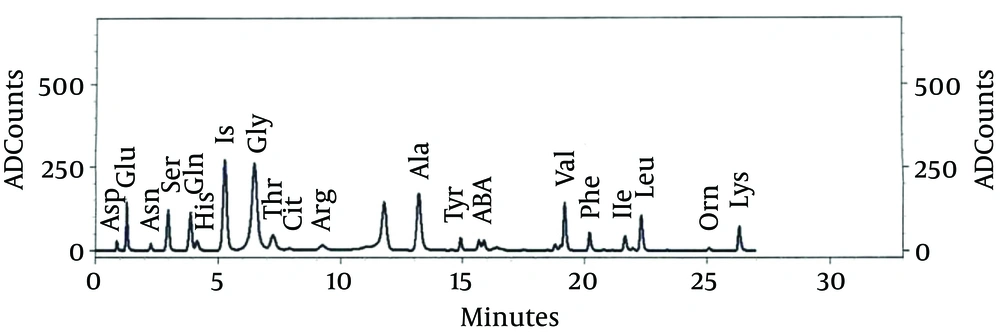
Due to lack of response to the treatment of seizures and continuation of jumping movements, phenobarbital was added to the treatment of epileptic patient. Chromatography amino acids of the patient’s CSF and blood were performed (Figures 1 and 2) (Table 2). In the metabolic tests, blood and urine glycine levels were reported as high. CSF glycine was 138.8 mM/L, plasma glycine was 809 μM/L; where CSF glycine ratio to plasma was 1.71. MRI was reported normal. With the diagnosis of hyperglycemia, she was treated by sodium benzoate (500 mg/kg), dextromethorphan (5 mg/kg) and L-carnitine. After the start of the treatment, the seizures, neurologic symptoms and the baby’s consciousness improved; and gradually by improving sucking reflex, gastric tube feeding changed to oral feeding. After two weeks, with seizure control, improved sucking reflexes and grasp, with continuation of treatment with oral sodium benzoate, dextromethorphan and L-carnitine, the patient was discharged.
| Name | Concentration, µM/L | |
|---|---|---|
| CSF | Blood | |
| ASp | 2.326 | 44.681 |
| Glu | 3.432 | 145.913 |
| Asn | 8.488 | 32.989 |
| Ser | 49.738 | 221.628 |
| Gln | 334.560 | 203.113 |
| His | 23.525 | 77.121 |
| IS | 1.000 | 1.000 |
| Gly | 138.886 | 809.291 |
| Thr | 53.671 | 175.039 |
| Cit | 4.828 | 22.494 |
| Arg | 13.941 | 53.611 |
| Ala | 33.110 | 377.765 |
| Tyr | 10.658 | 50.819 |
| ABA | 2.711 | 18.862 |
| Val | 19.119 | 148.455 |
| Phe | 13.688 | 87.795 |
| Ile | 7.909 | 49.742 |
| Leu | 20.474 | 127.735 |
| Orn | 8.411 | 79.593 |
| Lys | 29.724 | 180.263 |
Abbreviations: ABA, α-amino-n-butyric acid; Ala, alanine; Cit, citrulline; Arg, arginine; Asn, asparagine; Asp, aspartic acid; Gln, glutamine; Glu, glutamic acid; Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys, lysine Orn, ornithine; Phe, phenylalanine; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine.
3. Discussion
Fetal non-ketotic hyperglycemia is a lethal hereditary disorder with autosomal recessive inheritance. The prevalence of the disease is unknown, but it is common in southern Finland and has an incidence of approximately 1 in 1,000 live births (9). Patients with the disorder shortly after birth present symptoms such as encephalopathy, reduction of consciousness, loss of reflexes and finally seizures. These newborns are completely hypotoniac and have seizures completely resistant to therapy and ultimately die (10). The effects occur due to glycine aggregation in two areas of the brain with glycine receptors. Glycine receptors in the brain stem and spinal cord have inhibitory effects and therefore cause glycine aggregation and hypotonia and inhibit the accumulation. However, the receptors of the cerebrum and cerebellum region have stimulating effects, cause seizures, myoclonus and other neurological diseases and encephalopathy (11, 12). In order to diagnose this disorder, primarily glycine levels in the plasma and urine are determined and in case of high levels in the absence of ketonemia, the CSF glycine and the ratio of CSF glycine to plasma are measured. In the case of aggregation of large amounts of glycine in CSF compared to plasma (CSF glycine/plasma ratio more than 0.08) the diagnosis is confirmed. Golden standard of NKH to measure converting glycine enzyme (glycine cleavage enzyme) is in the liver biopsy. Glycine cleavage enzyme is a mitochondrial enzyme composed of four different proteins (P-protein, H-protein, T-protein and L-protein) coded by four genes, and mutations in any of these genes may end in NKH; most mutations are reported in P-protein gene (11, 13-15).
Imaging studies are not so effective to diagnose the disease; except that agenesis of corpus callosum on MRI in cases of reported disease. Differential diagnosis of this disease includes propionic academia, methylmalonic aciduria (MMA) and poisoning with sodium valproate that should be refused before confirming the diagnosis. Existence of acidosis in the first two diseases, and normalizing glycine after discontinuation of the drug can be involved in confirming or rejecting the differential diagnosis (16). To date, six patients are reported with fetal transient NKH that improved after a period; among them, five patients fully recovered and while reducing the levels of glycine, they did not have any neurologic complications and not significantly modified with glycine; despite modified glycine level, one patient got mental retardation. The mechanism of this phenomenon is not well described (17).
As mentioned, NKH is a fatal disease with no cures. Seizures in this disease are resistant to conventional anti-epileptics, and if not treated, the patient quickly gets ill and dies. It is also observed that if the treatment in such patients starts earlier, brain is less affected by the toxic effects of glycine and there will be fewer complications (11).
The treatment of this disease includes three drug groups: A) Plasma glycine-reducing agents, first group drugs reduce seizure frequency by reducing the level of plasma and CSF glycine, but they have no impact on neurologic status. In addition, they are unable to take CSF glycine level down to normal, the most important drug of this group is sodium benzoate and the usual dose of it in this disease is 150 - 750 mg/kg (10, 11, 18, 19). B) N-methyl-D-aspartate (NMDA) inhibitors; contrary to the first group, this one is not able to reduce levels of glycine. However, since glycine can stimulate NMDA receptors in the brain, inhibitors of this receptor have a significant impact on seizure control and improving neurological function. They can even modify abnormal electroencephalogram (EEG) waves of the brain. The most important drug in this group is dextromethorphan that can be prescribed at the dose of 0.25 - 5.35 mg/kg in the patients. Another drug of the group is ketamine, with a similar effect and can improve neurological function in patients, particularly in improving swallowing and sucking (10, 11, 20, 21). C) Competitive glycine inhibitors: This group can treat mild cases of the disease. The most effective drug of this group is strychnine that in mild cases improves patients’ neurologic function (11). Medications such as benzodiazepines and tryptamine did not affect the treatment of patients.
3.1. Conclusions
Since most of the symptoms of metabolic diseases in newborns are the same as the signs of sepsis or other common diseases of this period, and also early diagnosis and treatment of these illnesses, especially in diseases such as hyperglycemia can be influential in neural function of babies, it calls for serious attention and clinical vision of the doctor to stop mortality of such diseases by early consideration of serious complications. The mentioned patient was one of thousands patients in need of care and attention and her parents needed advice and transgenic study to have healthy children.