1. Background
Prostate cancer (PC) is one of the most frequently occurring cancers among men in Iran (1). It is predicted that the number of cases will almost double (to 1.7 million) by 2030 (1). Often, men with PC live longer post-diagnosis, with recent statistics indicating survival rates of up to 76.5% five years after diagnosis (2). The high rate of cancer survival is likely related to advances in early detection such as through the screening of prostate-specific antigen (PSA) and treatment modalities such as surgery (e.g., radical prostatectomy), androgen deprivation therapy (ADT), and radiation therapy (3). Regardless of the treatment strategies, PC survivors often experience a very high rate of morbidity caused by cancer and its treatments, particularly ADT. Examples of these side effects include significant reductions in various aspects of the quality of life, which often becomes more pronounced over time or with additional treatment (4). PSA is a widely used tumor marker in the screening and follow-up of PC. Because PSA measurement has such a crucial role in the management of PC, any urologic intervention that may cause PSA elevation must be detected (5). PSA is a serine protease produced by the epithelial cells of the prostate, and it serves to cause liquefaction of the seminal fluid. Galvao et al. (2010) reported that a combined resistance and aerobic exercise program, twice a week for 12 weeks, had no effect on PSA levels (6). Epidemiological studies have shown that chronic inflammation may be predisposed to certain kinds of cancer (7). Prostatic inflammation may represent an important factor influencing prostatic growth and the progression of symptoms (8). High-sensitivity C-reactive protein (hs-CRP), as an acute-phase plasma protein that increases during systemic inflammation, is one of the most frequently used inflammatory markers. CRP is produced primarily in the liver and is regulated by proinflammatory cytokines, especially interleukin-6 (9). Both chronic and acute inflammation may lead to events that can cause proliferation in the prostatic tissue through a variety of mechanisms like special oxidative stress (8). Inflammatory cells can become attracted to the prostate tissue microenvironment and can selectively promote the proliferation of prostate epithelial cells (10). Recent studies suggest that hs-CRP levels are positively associated with cancer (11). Therefore, it seems that high levels of CRP can promote the risk of PC and its metastasis. Interleukin-10 (IL-10), an anti-inflammatory cytokine, suppresses T1-helper cell responses and regulates lymphocytes, which are natural killer (NK) cells (12). In PC, an overexpression of IL-10 has been reported (12), but a reduced expression has been found when common polymorphisms in the promoters of the IL-10 gene were examined (13, 14). IL-6 expression is largely regulated by gene transcription. The tumor necrosis factor (TNF-α) is a strong promoter of IL-6. TNF-α is a cytokine that serves two conflicting functions: apoptosis or survival (15). A high expression of TNF-α has been correlated with the proliferation and survival of malignant cells, the stimulation of angiogenesis, and the metastases and alteration of the response to chemotherapeutic agents (16). Studies have shown that IL-6 involved in the regulation of immune responses causes cell growth rather than separation in many cell types. However, the effects of IL-6 on PC cells are contradictory (17). Physical activity and exercise training has been suggested as a key lifestyle intervention due to its potential to limit, and even reverse, some toxicities (18). Some authors examined the effects of exercise training in men with PC. Galvao et al. (2010) indicated that combined resistance and aerobic exercise improves muscle mass, strength, physical functions, and inflammatory markers in men undergoing ADT for PC (6). Segal et al. (2003) found that 12 weeks of resistance training in men that receive ADT reduces fatigue and improves the quality of life and overall muscular strength (19). Galvao et al (2006) showed that progressive resistance exercise has beneficial effects on muscle strength, functional performance, and balance in older men that receive ADT for PC and should be considered in preserving body composition and reducing treatment side effects (20). In addition, Culos-Reed et al. (2010) showed that physical activity effectively attenuates many of the side effects of ADT and should be recommended to prostate survivors as an alternative therapy. Findings from their study further support the role of resistance training in preserving composition and improving physical functions in PC patients (21). It seems that exercise training, especially resistance training, has functional benefits in patients with PC. Classic massage is one of the oldest and most regularly used complementary therapies, and it has become more and more popular in the past few years. It has been proven as an effective treatment for symptom relief in non-cancer patients (22), and cancer patients also reportedly benefit from massage therapy. They experience pain relief and a reduction in nausea, fatigue, and other cancer-related symptoms (23). Krohn et al. (2011) found that massage therapy is an efficient treatment for reducing depression in breast cancer patients. Insignificant results about stress, mood, and immunological parameters indicate that further study is needed to determine psychological and immunological changes under massage therapy (22). Tarhan et al. (2005) reported that prostatic massage increases serum-completed PSA concentration but to a lesser extent than the total PSA and free PSA (5). The effects of massage therapy in men with PC remain unclear.
2. Objectives
Examination of the effects of exercise training and massage in men with PC is new area of study in oncology and clinical exercise physiology. While a few studies have assessed the benefits of exercise or physical activity in PC patients, almost none have examined the effects of resistance training and prostatic massage on proinflammatory markers and PSA in men with PC. Therefore, this study aimed to investigate the effects of resistance training and prostatic massage on proinflammatory markers and PSA in males with PC.
3. Patients and Methods
Patients were selected from a urologic center. The eligibility criteria were as follows: (a) every patient was diagnosed with PC in the past month and (b) a physician’s clearance was obtained to participate in an exercise program consisting of massage and strength and flexibility exercises. The patients included 45 men with PC with a mean age of 59.5 ± 2.7 years. Approval was obtained from the appropriate ethical committees, and all patients were provided with written and informed consent prior to participation. Weight and body mass index were considered for homogeneity. They were randomized into either the resistance training intervention group (n = 15), the massage intervention group (n = 15), or the control group (n = 15). To define the statistics sample, a list of PC exclusion criteria was included. The exclusion criteria was as follows: hypertension, diabetes, bone metastatic disease, musculoskeletal disease, cardiovascular disease, liver disease, kidney disorders, neurological disorders, and immune system weakness that could inhibit exercise training or undertaking upper and lower limb exercise. Then, all patients obtained medical clearance from their physicians and completed physical activity readiness questionnaires.
3.1. Procedures
To reduce intervening factors that affect study results and to reduce the effects of sexual intercourse and dietary intake on inflammation and PSA, the patients were requested to refrain from sexual intercourse and from eating fast food or drinking caffeinated beverages. The ethics committee of Khorramabad University approved the study protocol with code No: SH/47/365, and informed consent was obtained from the subjects before treatment.
3.2 Intervention
Patients undertook progressive resistance training and massage three times per week for eight weeks. The resistance-training program included a 10-minute warm up using stretch movements. Then, training continued by rotating between eight stations for 50 – 60 minutes. Resistance training included the chest press, seated row, shoulder press, triceps extension, leg press, leg extension, and leg curl. Abdominal crunches were also performed. Resistance training in each session included three sets with 12 repetitions at 50 – 60% intensity of one maximum repetition. 40 – 60 seconds between stations and 90 seconds between sets was the optimal time to rest to ensure muscle recovery between stations and sets. The overload principle was performed by measuring one maximum repetition after each two-week training regimen. Patients performed cool-down exercises at the end of each training session for 10 minutes. Before the massage program, patients were asked not to eat food but to drink water and fill the bladder fully. A relaxing massage was performed at the patients’ convenience. Patients were requested to either sit on their knees and elbows or lie down on one side so that the knees stayed near the stomach. At first, caress and segment massages were performed along the prostate gland 5 – 8 times from the exterior to the interior and from the upper to the lower parts. Massage movements were directed from the outside toward the center. The process lasted 1 – 2 minutes. Then, a rubbing massage was applied on the mid-line prostate for 1 – 2 minutes so that the prostate wrinkled and its contents felt pressure. However, the pressure should not cause pain in the patients; otherwise, the massage must be stopped at any stage. The massage program was conducted 3 sessions per week for six weeks (24).
3.3. Usual Care
The control group did not participate in the resistance training or the massage program. They also did not participate in any exercise programs, but they did continue their current care. Since the patients were cancer survivors, this generally included a recommendation to call their oncologist or primary healthcare provider if any symptoms became problematic. Follow-up oncology appointments are placed at 2-month intervals and moved to annual visits after a year or after continued remission.
3.4. Blood Samples and Laboratory Analysis
Patient blood samples were taken, as much as 5 ml, in three stages (i.e., 48 hours before training, 48 hours after the last training session of the fourth week, and 48 hours after the last training sessions of sixth and eighth weeks) at 8 A.M. after 8 – 10 hours of fasting. The samples were taken in a supine position to measure the biochemical variables. In addition, control blood samples were collected along with the experimental groups. Serums were immediately centrifuged in 3000 rpm and kept in a freezer at 70˚C until analysis. The serum levels of PSA, IL-6, and CRP were measured using the ADALTI PSA (Italy), SANGUIN (the Netherlands), and RANDOX (England) kits respectively. Further, IL-10 and TNF-α were measured by the QUNATIKINE (USA) kit. All biochemical variables were assayed by the ELISA method.
3.5. Statistical Methods
To describe the obtained data for all the variables, frequency, mean, and standard deviation were used (Table 1). The Kolmogorov-Smirnov test was used to define the normality of data. Given the normal distribution of data, repeated measures analysis of variance (ANOVA) was used to analyze the data for intra-group comparison of PSA, CRP, TNF-α, IL-6, and IL-10. A Bonferroni follow-up test was used to see if there were any significant differences. Furthermore, an independent t-test was used to compare the mean for both the experimental group and the control group in mid- and post-test stages (P ≤ 0.05).
4. Results
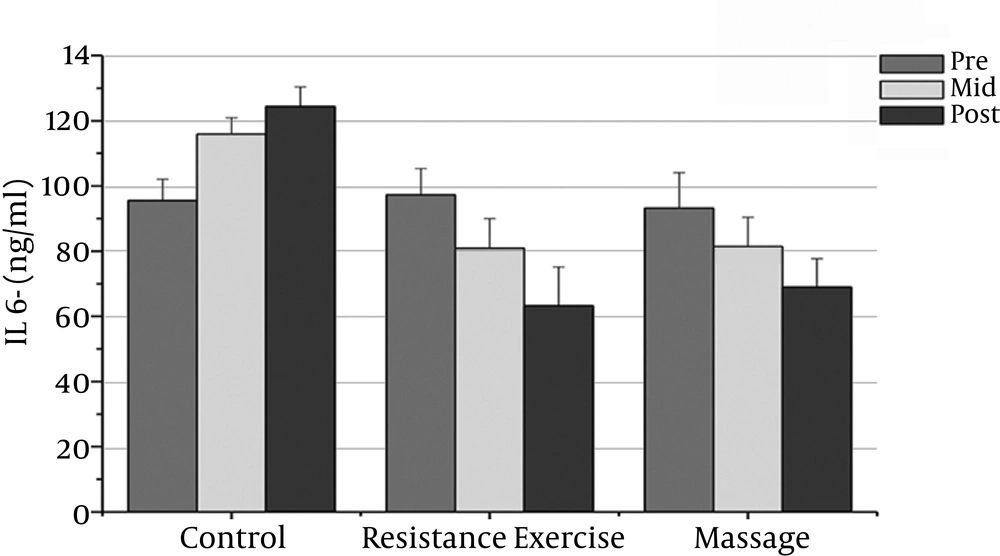
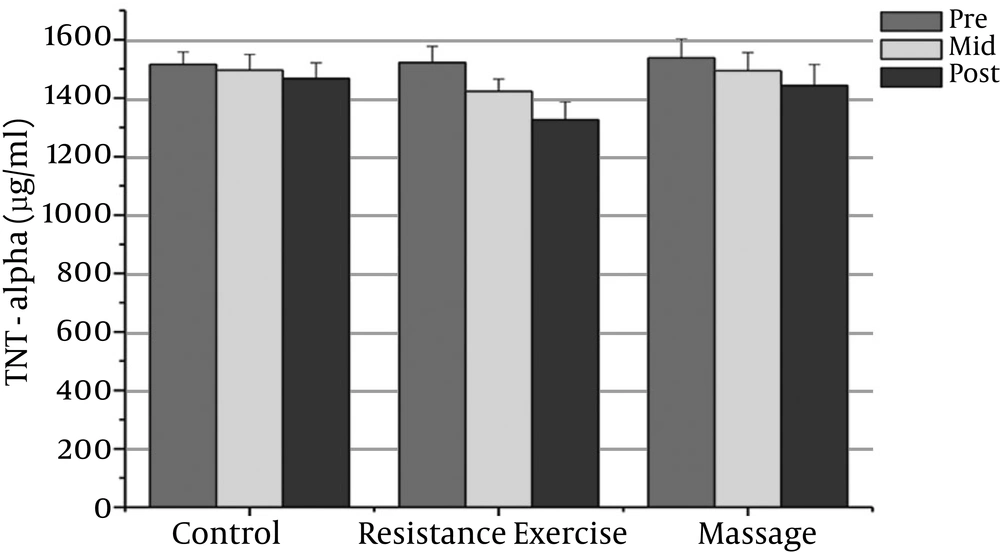
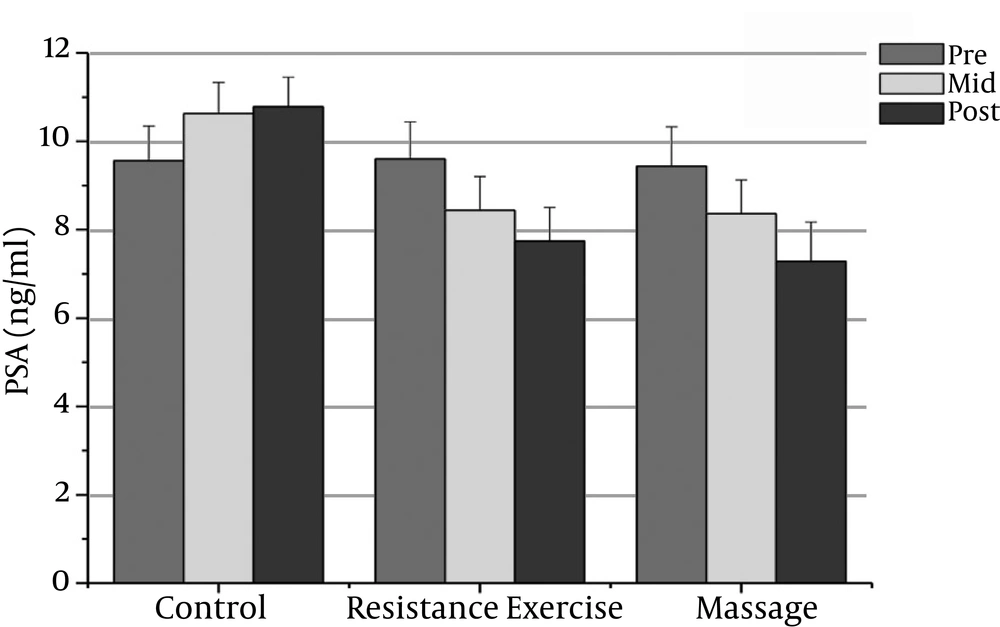
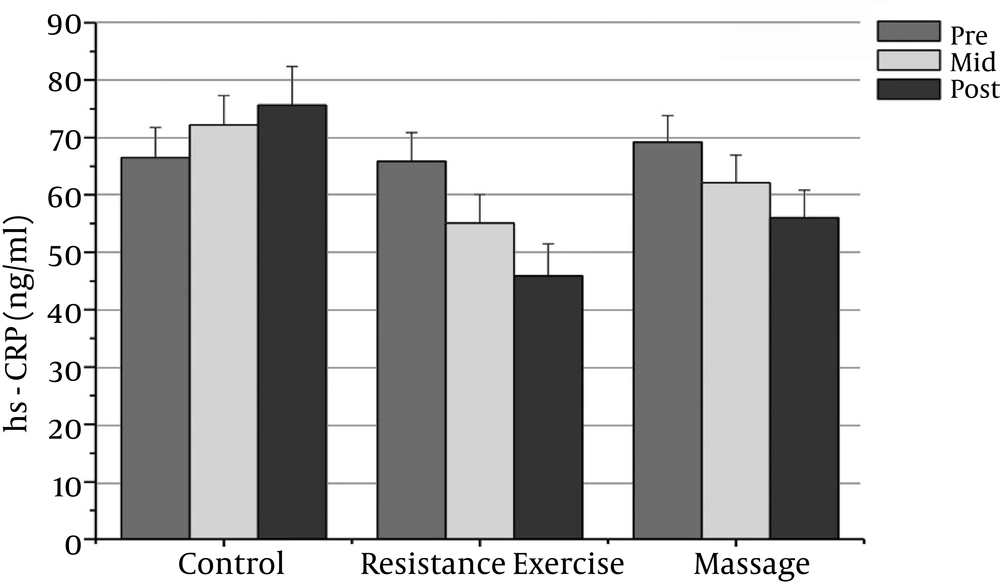
The normality of patients has been presented as the demographic characteristics at the baseline in Table 1. The findings showed that there were significant differences in PSA, CRP, TNF-α, IL-6, and the IL-10 between the groups (P = 0.000). The PSA serum levels in the massage group reduced significantly after the sixth week compared to the baseline (P = 0.000). In addition, the CRP serum levels in the massage group reduced significantly after the sixth week of massage treatment. The Massage group’s TNF-α levels reduced significantly after the sixth week compared to before massage therapy (P = 0.000). The massage group’s IL-6 levels reduced significantly after the sixth week compared to before massage therapy (P = 0.000).
| Group Massage | Group Resistance | Group Control | P-Value | |
|---|---|---|---|---|
| Age, y | 60.10 ± 2.76 | 61.23 ± 1.41 | 61.15 ± 1.68 | 0.95 |
| Weight, kg | 69.45 ± 2.70 | 70.15 ± 2.34 | 70.43 ± 1.56 | 0.97 |
| Height, cm | 169.43 ± 1.77 | 170.19 ± 2.02 | 171.26 ± 1.87 | 0.59 |
| BMI, kg/m2 | 21.98 ± 1.19 | 22.59 ± 1.78 | 22.10 ± 1.38 | 0.88 |
Normality of Patient Distribution as Demographic Characteristics at the Baselinea
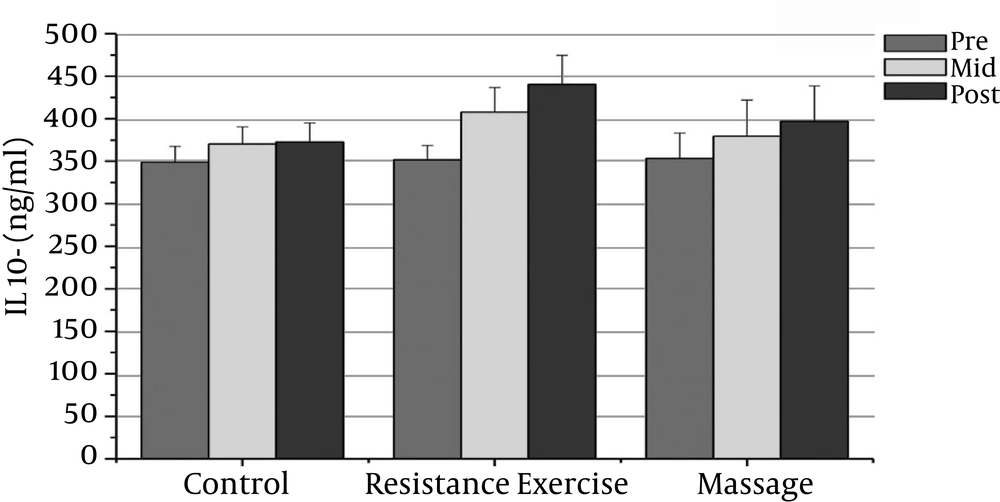
However, the massage group’s IL-10 levels increased significantly after the sixth week compared with the baseline (P = 0.000). There was a significant decrease in PSA, CRP, TNF-α, and IL-6 after the fourth week compared with the baseline (P = 0.000).
The resistance group’s PSA levels reduced significantly after the eighth week compared with the baseline (P = 0.000). The resistance group’s CRP levels declined significantly after the eighth week compared with the baseline (P = 0.000). The resistance group’s TNF-α levels decreased significantly after the eighth week compared to before training (P = 0.000). The resistance group’s IL-6 rates reduced significantly 48 hours after the eighth week compared to before training (P = 0.000). The resistance group’s IL-10 levels increased significantly 48 hours after the eighth week (P = 0.000). There was a significant decrease in PSA, CRP, TNF-α, and IL-6 after the fourth week compared with the baseline (P = 0.000).
The results showed significant differences in PSA, CRP, and IL-6 between the massage and control groups. The PSA serum levels in the massage group was significantly different 48 hours after the sixth week compared with the control group (P = 0.000). In addition, there were significant differences between the massage and control groups in the CRP and IL-6 levels after the sixth week (P = 0.000). There were no significant differences between the massage and control groups in TNF-α or IL-10 levels.
The PSA and CRP levels in the resistance group were significantly different after the eighth week compared with the control group (P = 0.000). The TNF-α and IL-6 levels in the resistance group were significantly different after the eighth week compared with the control group (P = 0.000). The resistance group IL-10 levels increased significantly after the eighth week compared with the control group (P = 0.000).
| Massage Group | Resistance Group | Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Intervention | Fourth Week of Intervention | 48 Hours After Intervention | Before Intervention | Fourth Week of Intervention | 48 Hours before Intervention | Before Intervention | Fourth Week of Intervention | 48 Hours before Intervention | |
| PSA (ng/ml) | 9.44 ± 0.89 | 8.36 ± 0.76t | 7.28 ± 0.89t | 9.60 ± 0.84 | 8.44 ± 0.76t | 9.56 ± 0.79f | 9.56 ± 0.79 | 10.63 ± 0.7 | 10.78 ± 0.67 |
| CRP (mg/l) | 69.23 ± 4.62 | 62.13 ± 4.82t | 56.05 ± 4.78b | 65.87 ± 5.06 | 55.11 ± 4.9 | 45.91 ± 5.54b | 66.51 ± 5.26 | 72.21 ± 5.10 | 75.64± 6.76 |
| TNF-a (pg/ml) | 1537.13 ± 64.49 | 1493.93 ± 64.4 | 1442.39 ± 73.10 | 1521.80 ± 55.63 | 1423.23 ± 1.4f | 1326.06 ± 62.36t | 1515.53 ± 42.12 | 1496 ± 53.82 | 1467.26 ± 53.33 |
| IL-6 (pg/ml) | 93.20 ± 10.91 | 81.40 ± 8.59t | 81.40 ± 8.95t | 97.26 ± 8.08 | 80.93 ± 9.l0t | 63.20 ± 11.82t | 95.53 ± 6.65 | 115.93 ± 5.02 | 124.64 ± 6.03 |
| IL-10 (pg/ml) | 353.4 ± 29.69 | 379.53 ± 42.l3t | 379.53 ± 42.l3t | 351.73 ± 16.94 | 407.667 ± 29.05b | 440.33 ± 34.164b | 348.86 ± 18.66 | 374.40 ± 20.19 | 372.863 ± 22.26 |
5. Discussion
The aim of the present study was to investigate the effects of eight weeks of regular resistance training and six weeks of prostatic massage on PSA levels and proinflammatory markers (CRP, IL-6, TNF-α, and IL-10) in males with PC. It should be noted that only a few studies have investigated the topics related to this study; therefore, comparing the current study’s findings with prior results is difficult. However, previous reports have shown that inflammation causes PC (25). The present study demonstrated that four (P = 0.00) and six (P = 0.00) weeks of prostatic massage improve PSA in patients with PC, and this is inconsistent with other studies examining the individual effects of prostatic massage after ADT, chronic prostatitis, and radiation therapy (5, 26). Likewise, it has been reported that free PSA is the most increased form after prostatic manipulation (5, 27). In addition, the current study demonstrated that four (P = 0.00) and eight (P = 0.00) weeks of resistance training reduce PSA. The findings are consistent with the findings of Segal et al. (2003) (19) and other studies examining the individual effects of resistance training after AST and radiation therapy. Resistance training had no impact on PSA levels in previous studies (20, 28). These contradictions may be due to the duration of the massage session and the total duration of the protocols. It may also be due to the reduced IL-6 levels because signaling pathways activated by cytokines, such as IL-6 and IL-4, have been shown to activate the androgen receptor and stimulate PSA expression (29). In addition, other findings of the present study indicated that four (P = 0.00) and six (P = 0.00) weeks of prostatic massage increase IL-10 in patients with PC. This is consistent with the study of Krohn et al., who found a significant increase in IL-10 after massage therapy in primary breast cancer patients (22). Six weeks of prostatic massage may enhance immune functions, even if it is temporary and transient. Further, the results of this study demonstrated that four (P = 0.00) and eight (P = 0.00) weeks of resistance training elevates IL-10. Experimental studies (12) and a large case-control study (14) suggest that IL-10 plays a role in PC. However, another study concluded that IL-10 does not contribute to PC progression (18). Richardsen et al. found no difference in IL-10 expression between non-metastatic and metastatic primary tumors, in neither tumor cell areas nor tumor stromal areas (12). The apparently inconsistent data may reflect the dual biological function of IL-10 as an anti-inflammatory (potentially cancer-promoting) and anti-angiogenic (potentially cancer-inhibiting) cytokine in relation to the biological patterns of PC development and progression (12). It is possible that resistance training programs have antioxidant effects and increase IL-10 and improve the immune system and liver functions. This study showed that four (P = 0.00) and six (P = 0.00) weeks of prostatic massage significantly reduce TNF-α in patients with PC. This finding is consistent with the study of Krohn et al., who found a significant reduction in TNF-α after massage therapy in primary breast cancer patients (22). Elevated pretest cytokine secretion is responsible for the activation of the cellular immune system; therefore, it is crucial in tumor immunity. For example, NK cell activity and cytotoxicity have been found to increase after massage therapy (30). Elevated NK cell, cytotoxic T lymphocyte, and overall lymphocyte counts have also been discovered after massage therapy (22, 23). Other results of the current study demonstrated that four (P = 0.00) and eight (P = 0.00) weeks of resistance training reduce TNF-α in patients with PC. Likely, a reduction in TNF-α after resistance training is related directly to the reduction of cytokine production in the adipose tissue, muscle cells, and mononuclear cells and indirectly through weight loss and improvement of endothelial cells. Conversely, four (P = 0.00) and eight (P = 0.00) weeks of resistance training and four (P = 0.00) and six (P = 0.00) weeks of prostatic massage reduce IL-6 in men with PC. IL-6 has an important role in the regulation of immune reactions, cell growth, and differentiation in many cell types. TNF-α stimulates focal adhesion kinase activity, and focal adhesion kinase signaling is important for TNF-α-induced IL-6 mRNA and protein expression in prostate, lung, breast, and neural tumor cells by a mechanism that involves ERK/MAPK signaling activation (15). Thus, in this study, reduced TNF-α is the reason for a reduction in IL-6. Resistance training and prostatic massage, possibly through stimulating pressure receptors and impressing nerves in internal organs, the limbic system, and the vessel wall, reduce sympathetic activity and increase parasympathetic activity, eventually reducing inflammation and IL-6 levels. Four (P = 0.00) and eight (P = 0.00) weeks of resistance training significantly reduce CRP protein levels in men with PC, and four (P = 0.00) and six (P = 0.00) weeks of prostatic massage can significantly improve CRP in patients with PC. These findings are consistent with a study by Galvao et al. (6). The systemic inflammatory response is caused by the stimulation of inflammatory cytokines, and interlukin-6 (IL-6) is the potent inducer of CRP production (31). Therefore, CRP reductions are caused by decreased IL-6. Elevated hs-CRP is associated with progressive diseases and decreased survival in patients with several cancers including esophageal, prostate, colorectal, liver, pancreatic, urinary bladder, kidney, ovarian, and cervical cancers (11). CRP, a representative acute-phase reactant, could indicate the aggressiveness of PC (31). Resistance training and prostatic massage reduce stress and CRP levels. The levels of inflammatory markers in patients with PC are high. Resistance training and massage programs can reduce PSA levels and proinflammatory markers in males with PC. Furthermore, the application of resistance training and massage can be a complementary treatment for men with PC.